Abstract
Onychogryphosis is one of the main clinical findings in dogs with visceral leishmaniasis (VL); however, research focusing on the subungual area of infected dogs is scarce. This study aims to assess the subungual area of dogs with VL that presented or not onychogryphosis by means of histopathological analyses and immunohistochemical studies (parasite burden). The third digit of the thoracic and pelvic limbs of Leishmania infantum naturally infected dogs was collected regardless of sex, breed or age. The animals were split into two groups, dogs with onychogryphosis (G1; n=7) and without onychogryphosis (G2; n=9). The digits were evaluated in four areas (dorsal epidermis/dermis, ventral epidermis/dermis, dorsal matrix/dermis and ventral matrix/dermis). All lesions observed (mononuclear inflammatory infiltrate, vacuolar degeneration of basal keratinocytes, dermoepidermal clefting and pigmentary incontinence) were present in both groups, being more severe in the digits of G1 group. Immunostaining of the amastigote forms of Leishmania infantum were observed in the different areas of the digit, with statistical difference between the dorsal epidermis/dermis area and the dorsal matrix/dermis of G1 group. In conclusion, the main histopathological alteration of the digit of dogs with VL is mononuclear inflammatory infiltrate and parasite burden, especially in cutaneous tissue adjacent to the nail matrix. This aspect can influence the onychogryphosis development, due to the presence of the parasite and by inflammatory mediators released in the nail microenvironment.
Author Contributions
Academic Editor: Mohammed Elmetwally, Assistant Prof of Theriogenology, Egypt.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Paulo Henrique Leal, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Visceral leishmaniasis (VL) is an anthropozoonosis broadly disseminated around the world, which affects human beings, dogs and wild animals 1 and whose etiologic agent in the American continent is the protozoan Leishmania infantum 2. Mosquitos of the species Lutzomyia longipalpis and Lutzomyia cruzi are the vectors involved in disease transmission 3. The kennel tick Rhipicephalus sanguineus may host the parasite, but its role in the transmission of the parasite is yet to be proven 4.
The clinical signs of VL are varied, such as generalized lymphadenomegaly, progressive weight loss, splenomegaly, hepatomegaly, skin alterations, eye lesions, kidney diseases, neurological disorders, and onychogryphosis 5.
Onychogryphosis is the excessive growth of nails, and is among the main clinical findings in dogs with VL, being reported in the proportions of 63.1% 6, 40% 7 and 55% 8. Some authors correlate nail overgrowth to the presence of the parasite in the nail matrix 2. In one study, a weak amplification of the parasite DNA was detected in the digit of a dog with VL 7. However, the literature investigating onychogryphosis pathogens in dogs with VL is scarce.
Therefore, the aim of this study was to assess the subungual area of the thoracic and pelvic limbs of dogs with VL that presented or not onychogryphosis by means of histopathological analyses and immunohistochemical studies. Concurrently, the severity of lesions in different sites of the subungual area was compared, and the findings were correlated with the presence of onychogryphosis and parasite load at the site.
Material and Methods
The animals used in this study came from the Zoonoses Control Center of the city of Araçatuba (São Paulo, Brazil), an endemic region for VL. These dogs underwent euthanasia according to Brazilian legislation 9. The third digit of the right thoracic and pelvic limbs of 16 (n=32) Leishmania infantum naturally infected dogs was collected. There was no preference in regards to sex, breed or age. The dogs were selected on the basis of serum testing (ELISA) or fine needle aspiration of the popliteal lymph node for parasite visualization. These animals were split into two groups, dogs with onychogryphosis (G1; n=7) and without onychogryphosis (G2; n=9).
Fragments of the digits were fixated in 10% formalin, tamponated phosphate (0.15 Molar), pH 7.2, for 48 hours. After fixed, the entire digits were decalcified in 10% nitric acid solution for 8 days. Next, a median sagittal section was performed on the digits with a microtome disposable blade (Figure 1). The digits were washed in running water for 15 minutes, in baking soda for two hours and again in running water for four hours. Subsequently, the tissues were dehydrated and processed until they were embedded in paraffin according to the routine histological technique. They were then cut in 5μm thick pieces and stained with hematoxylin and eosin (H&E), modified protocol by 10.
Figure 1.Median sagittal section of the third thoracic digit of a dog with visceral leishmaniasis. Note the different areas of canine digit. (1) Flexor digitorum profundus muscle tendon, (2) Part of the third digit middle phalanx, (3) Extensor digitorum profundus muscle tendon, (4) Dorsal epidermis/dermis, (5) Dorsal matrix/dermis, (6) Nail wall, (7) Part of the third digit distal phalanx, (8) Ventral matrix/dermis, (9) Ventral epidermis/dermis (footpad).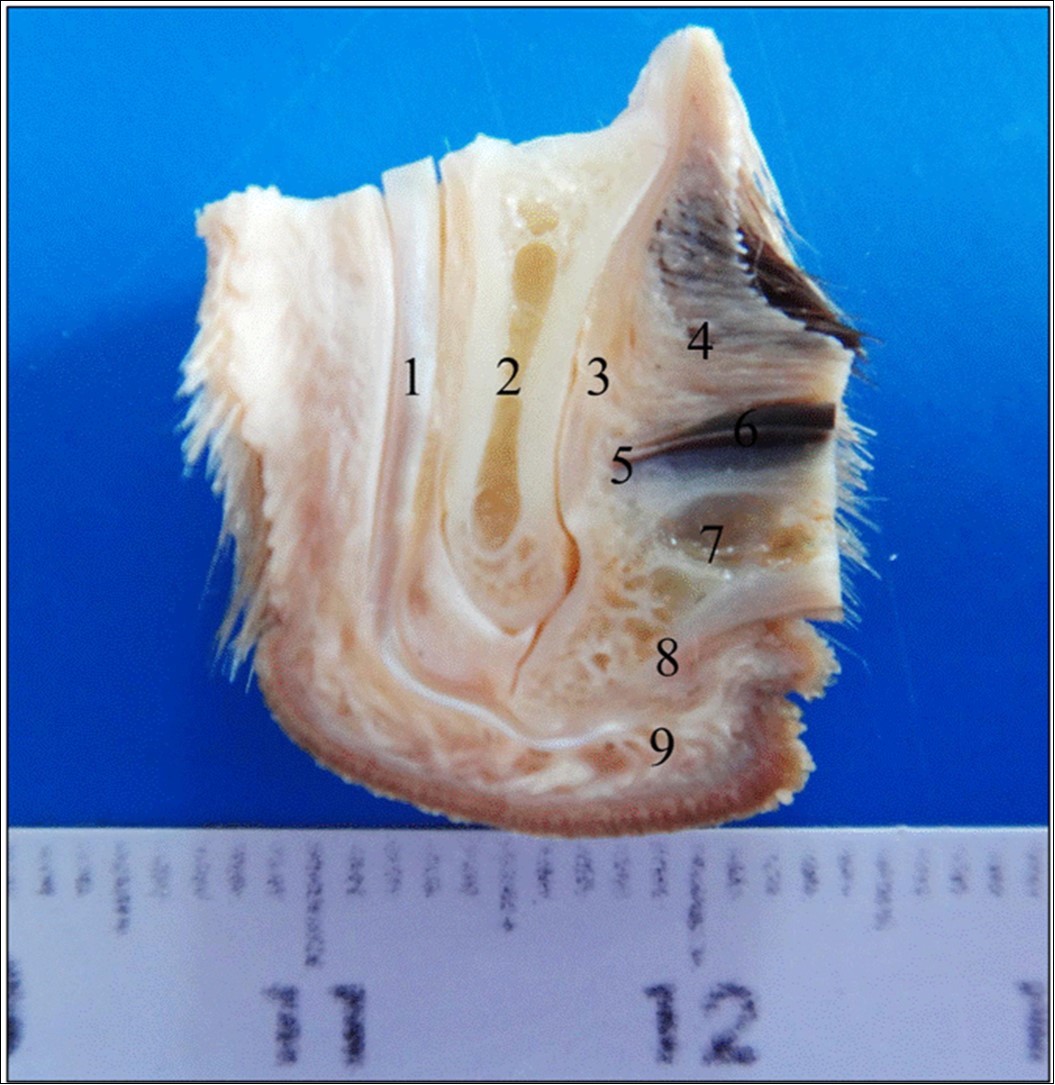
The digit histopathological findings were scored on a scale of 0-3 (0-absent, 1-mild, 2-moderate, 3-marked). Each area of the digit was scored: dorsal epidermis/dermis, dorsal matrix/dermis, ventral matrix/dermis and ventral epidermis/dermis (ventral claw bed), as described by 7.
The determination of the parasite load in the different areas of the digit was made according to the immunohistochemical method modified by 11. The tissues were deparaffinized in a dry oven at 60ºC for one hour and subsequently hydrated in solutions with decreasing concentrations of alcohol until being washed in distilled water. After, endogenous peroxidase blocking was performed with methanol solution (Synth) and 8% hydrogen peroxide (30 volumes, Synth) for 30 minutes at room temperature and protected from light. Blocking of nonspecific proteins was made with a commercial product (Protein Block, DakoCytomation, Code X0909), for 30 minutes in a humid chamber at room temperature. The serum of a VL-positive dog was used as the primary antibody at a 1:200 dilution, with two hours incubation at room temperature. The specimens were then incubated with the peroxidase-bound polymer complex (kit Advance HRP, DakoCytomation, Cód. K4068). Between each of the stages described the specimens were bathed in distilled water and in Tris HCl buffer solution, pH 7.4. For the reaction to be visualized, DAB chromogen (3,3'-diaminobenzidine – DakoCytomation, code K3468-1) was used. The tissues were counterstained with Harris hematoxylin and the mounting medium was Entellan (Merck). The lymph node of a VL-positive dog was used as positive control. Negative control was done with antibody diluent (Dakocytomation, code S3022) to replace the primary antibody.
The density of parasitic cells per animal was determined with the selection of eight microscopic fields of the digits with an objective lens of 40x (area of 0.19625 µm2) in a Nikon E200 microscope, Motic 2.5 megapixels digital camera. Mean immunomarker values in the fields of both groups, G1 and G2, were compared; comparison was also made within each group for the different areas of the digits, and for each limb (thoracic and pelvic).
The mean parasite load per animal was evaluated in each group (G1 and G2) and in the different areas of the digit within each group. The resulting observations were subjected to variance analysis (ANOVA), using the SAS computer software (SAS 9.1, SAS Institute, Cary, NC, USA). The assumptions of normal distribution of the residuals and homogeneity of the variances were checked. To meet these assumptions, the data were transformed into log10 (G1 group) and square root (1/SQRT) for comparison between groups and also for the different areas of the digit (G2). The comparisons of the mean parasite were made using Tukey’s test (p<0.05). Comparison between the limbs (thoracic and pelvic) was made using the nonparametric Mann-Whitney test and the software GraphPad Prism (version 6.01) was used (P<0.05).
Results
The main histopathological alterations (Table 1) in the four areas of the digit were mononuclear inflammatory infiltrate (Figure 2A) consisting of lymphocytes, plasmocytes and macrophages containing intracytoplasmic amastigotes of Leishmania infantum (Figure 2B); vacuolar degeneration of basal keratinocytes; dermoepidermal clefting (Figure 2C) and pigmentary incontinence (Figure 2D).
Table 1. Main histopathological alterations found in the different sites of the subungual area of the thoracic and pelvic limb digits of dogs with visceral leishmaniasis.| Type of alteration | Digit site | G1 Group | G2 Group | ||
| Frequency (%) | Severity (median) | Frequency (%) | Severity (median) | ||
| II* | DE/D | 14/14 (100) | 0-3 (2) | 13/18 (72.2) | 0-3 (2) |
| DM/D | 9/14 (64.2) | 0-2 (1) | 8/18 (44.4) | 0-3 (0) | |
| VM/D | 12/14 (85.7) | 0-3 (1.5) | 8/18 (44.4) | 0-3 (1) | |
| VE/D | 14/14 (100) | 0-3 (1) | 12/18 (66.6) | 0-3 (1) | |
| VD** | DE/D | 9/14 (64.2) | 0-2 (1) | 6/18 (33.3) | 0-2 (0) |
| DM/D | 5/14 (35.7) | 0-3 (0) | 6/18 (33.3) | 0-2 (0) | |
| VM/D | 6/14 (42.8) | 0-2 (0) | 7/18 (38.8) | 0-2 (0) | |
| VE/D | 8/14 (57.1) | 0-3 (1) | 7/18 (38.8) | 0-3 (0) | |
| Clefting D/E† | DE/D | 3/14 (21.4) | 0-1 (0) | 4/18 (22.2) | 0-3 (0) |
| DM/D | 9/14 (64.2) | 0-3 (1.5) | 7/18 (38.8) | 0-3 (0) | |
| VM/D | 6/14 (42.8) | 0-3 (0.5) | 5/18 (27.7) | 0-2 (0) | |
| VE/D | 3/14 (21.4) | 0-3 (0) | 5/18 (27.7) | 0-3 (0) | |
| PI‡ | DE/D | 5/14 (35.7) | 0-3 (0) | 2/18 (11.1) | 0-3 (0) |
| DM/D | 5/14 (35.7) | 0-3 (0) | 7/18 (38.8) | 0-3 (0) | |
| VM/D | 7/14 (50) | 0-3 (1) | 5/18 (27.7) | 0-3 (0) | |
| VE/D | 10/14 (71.4) | 0-3 (1) | 9/18 (50) | 0-3 (0.5) | |
Figure 2.Photomicrographs of histopathological alterations in the different digit areas of dogs with visceral leishmaniasis. (A) Inflammatory infiltrate (*) diffusely dispersed in dorsal epidermis/dermis (bar=100µm). (B) In the same area, inflammatory infiltrate containing macrophages with Leishmania spp amastigotes (arrows and detail, bar=20µm). (C) Inflammatory infiltrate (*) and pigmentary incontinence (arrow) in the dorsal matrix/dermis (bar=100µm). (D) Inflammatory infiltrate (*) and marked dermoepidermal clefting (arrow) in the ventral matrix/dermis (bar=100µm). Hematoxylin and Eosin.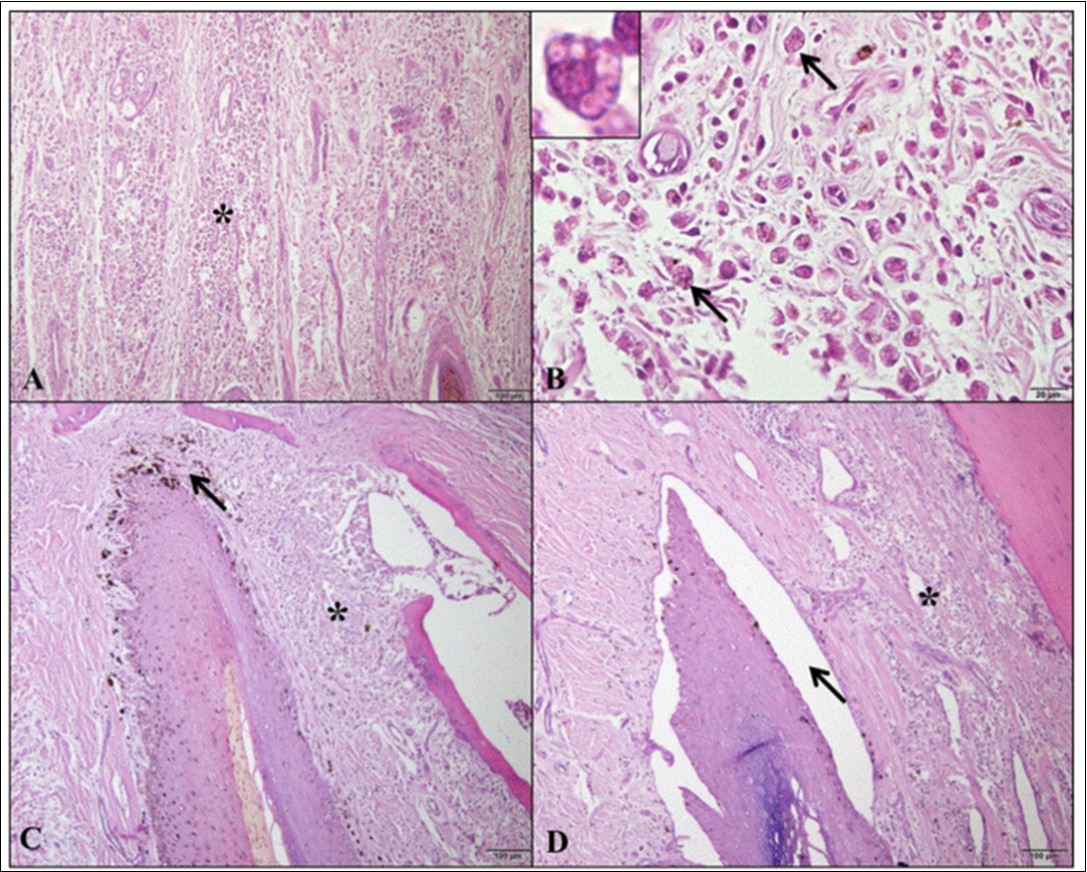
Immunohistochemical analysis identified cytoplasmic immunomarkers for amastigote forms of Leishmaniainfantum in the different areas of the digit (Figure 3).
Figure 3.Photomicrographs of Leishmania infantum amastigote immunomarkers in the digit of dogs with visceral leishmaniasis. (A) Note the detection of parasite in the macrophage cytoplasm in the dorsal epidermis/dermis (*; detail; bar=50µm). (B) Parasitized macrophages in the dorsal matrix/dermis (*; detail; bar=100µm). (C) Presence of parasite in the ventral matrix/dermis (*; detail; bar=100µm). (D) Infection with parasitized macrophages in ventral epidermis/dermis (footpad) (*; detail; bar=100µm). Peroxidase-bound polymer complex.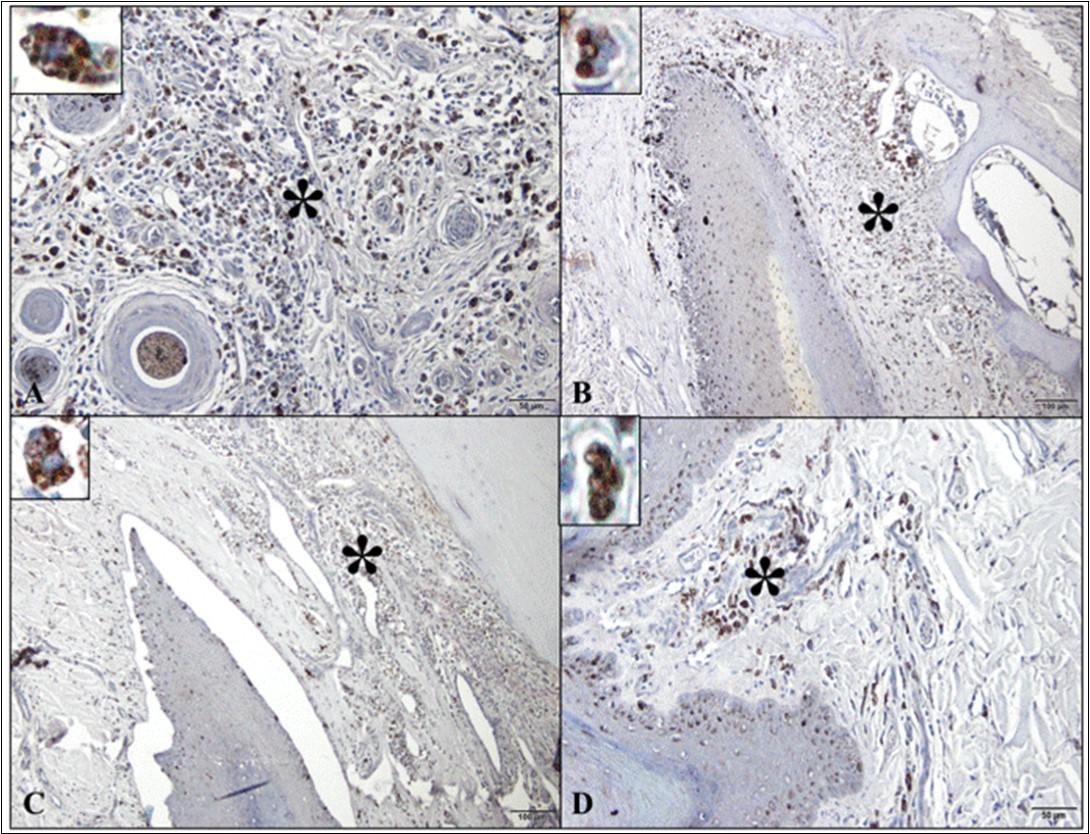
Comparison between thoracic (p=0.739) and pelvic (p=0.450) limbs did not reveal a difference between the groups (Mann-Whitney test). Comparison between the groups 1 and 2, regarding each area, did not reveal significant differences (p=0.0538; Tukey’s test / Figure 4). In the comparisons of areas, for the G1 group there were significant differences (p=0.0085, Tukey’s test / Figure 5) and for the G2 group did not showed significant differences (p=0.3709, Tukey’s test). In the G1 group, the dorsal epidermis/dermis and dorsal matrix/dermis showed significant differences in comparison to others digit areas (Figure 5).
Figure 4.Parasite load means ascertained from the comparison between the groups of dogs with onychogryphosis (G1) and without onychogryphosis (G2). Tukey’s test (p=0.0538).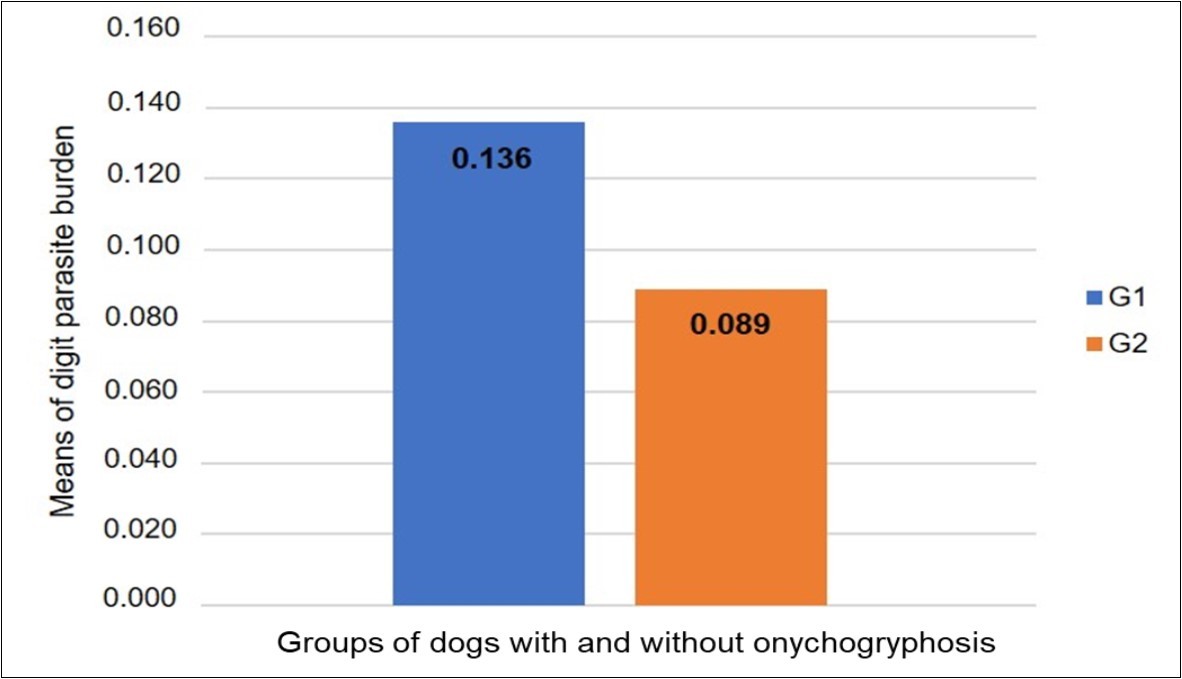
Figure 5.Parasite load means ascertained from the comparison between the different digit areas of animals with onychogryphosis (G1), that showed significant differences between digit areas (p=0.0085). Tukey’s test. DE/D = dorsal epidermis/dermis; DM/D = dorsal matrix / dermis; VM/D = ventral matrix / dermis; VE/D = ventral epidermis / dermis.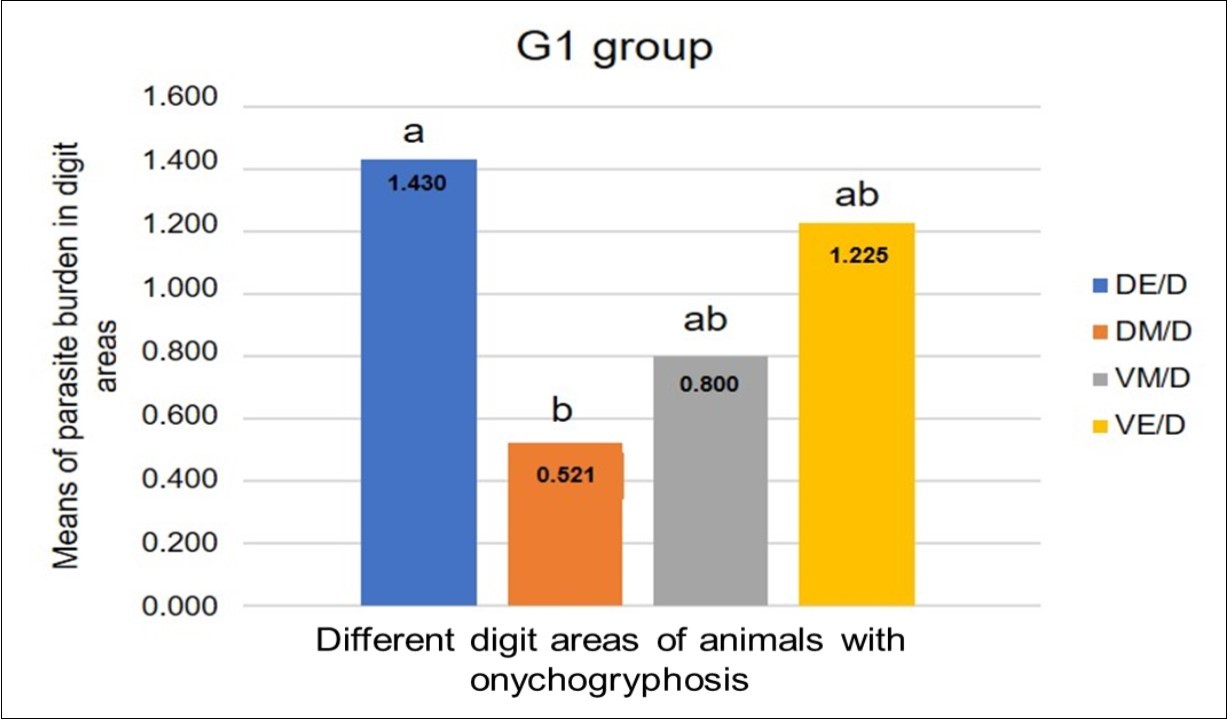
Discussion
Onychogryphosis in dogs may be related to bacterial, fungal or parasitic-induced alterations, trauma, systemic, proliferative and immunomediated diseases 12. In addition, this nail disease may be associated with paronychia from different causes. Paronychia is an infection around the nail that may be presented as tumefaction, hyperemia, alopecia and ulcers at the site 13, 14. In this study no dogs with onychogryphosis presented paronychia, which supports the report by 7.
Among the histopathological findings at the subungual area of the dogs investigated in this study, chronic dermatitis was representative in the four digit areas of dogs with onychogryphosis, and a little less frequent in dogs without onychogryphosis. Some authors report that onychogryphosis seems to be related to a local inflammatory process 2. In the G2 dogs (without onychogryphosis), the inflammatory process was also seen in the subungual area; these animals, however, did not develop this nail disease. 14 highlighted that onychogryphosis only becomes clinically evident after progression of the tissue injury for some time, and inflammatory response in the nail dermis.
The histopathology assessment of dogs with healthy nails made possible the observation of intranuclear vacuoles of basal cells 15; this is different from the vacuolar degeneration of basal keratinocytes found in this study. The vacuolar degeneration of basal keratinocytes was more frequent and severe in animals of the G1 group (with onychogryphosis). This finding was different than the finding of 7, who found the same alteration in 50% of the animals they had investigated, regardless of the presence of onychogryphosis. This alteration is a consequence of lysis of basal keratinocytes, which are accountable for skin hemostasis. These vacuolized cells may cause a fissure between epidermis and dermis 16.
Dermo epidermal clefting was more frequent in animals of the G1 group, particularly in the dorsal matrix/dermis and (64.2%) and ventral matrix/dermis (42.8%). 15 have also observed this alteration in healthy dogs, being 40% in the dorsal matrix/dermis and 15% in the ventral matrix/dermis, which was attributed to processing artifacts. The sample decalcification time of these authors and also of the authors of this study was similar (eight days). However, 7 had used a longer decalcification time (40 days) to decrease artifacts of the specimen cuts and found alteration frequency similar to the one of our study. This suggests that this alteration may occur in canine VL cases due to weakening of the dermoepidermal junction from the inflammatory process.
The highest frequency of pigmentary incontinence was in animals of the G1 group, while 7 have observed it in VL dogs with or without onychogryphosis. 17 have also observed this alteration in exfoliative dermatitis of VL dogs. For 16 pigmentary incontinence is due to a non-specific inflammatory lesion, and is seen in diseases that damage basal cells or melanocytes, such as systemic lupus erythematosus. Hence, this abnormality can be observed in VL inflammatory response.
Immunomarking of Leishmania infantum amastigotes was evident in the four regions of the digit, particularly in the dorsal epidermis/dermis, followed by ventral epidermis/dermis, ventral matrix/dermis and dorsal matrix/dermis. Comparison between the digit areas of the G1 group showed high means for dorsal epidermis/dermis and this area was statistical significant to dorsal matrix/dermis. These differences may suggest that the dorsal epidermis/dermis is an easy access area for the insect vector, which results in a higher parasite load.
In this study, the immunohistochemical analyses demonstrated Leishmania infantum amastigote forms in the subungual area of VL dogs. For 18, the decalcification process, even if for a short period of time (two days), leads to protein denaturation and possible immunohistochemical technique failure. 7 impute the failure of H&E and immunohistochemical analyses in their studies to the protracted decalcification process (40 days). Notwithstanding, the authors were able to amplify the DNA weak bands of Leishmania spp. (PCR) in the subungual area of VL dogs. These authors, however, did not inform the fixation time, sample size and the decalcifying agents. Both studies have used formic acid as a decalcifying agent, while this study used nitric acid and baking soda as a neutralizing agent of the reaction. It is thus suggested that immunomarking flaws of other studies is related to differences in the decalcification protocol.
In summary, the histopathological lesions and presence of the parasite were more pronounced in the dorsal epidermis/dermis and the ventral epidermis/dermis regions. The nail matrix had discrete alterations and a lower parasitic load when compared to the other areas of the canine digit. Possibly, the presence of the inflammatory mediators released in the cutaneous tissue adjacent to the nail matrix, in response to the presence of the parasite, may have influenced the exaggerated growth of the nail in the group of dogs with onychogryphosis (G1).
Conclusion
Chronic inflammatory infiltrate was the main histopathological finding of the subungual area of VL dogs. The dorsal epidermis/dermis region was the most affected in the dogs with onychogryphosis. This nail disease does not seem to be associated only with the parasite, but also with the inflammatory microenvironment of the adjacent cutaneous tissue, which may contribute to the development of onychogryphosis due to the release of inflammatory mediators.
Compliance with Ethical Standards
The present study was evaluated and approved by the University of the State of São Paulo Ethics Committee on Animal Use (CEUA-UNESP), in Jaboticabal, registered under nº 11976/15.
Acknowledgements
Financial support for this study was given by the Sao Paulo Research Foundation (Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP; no. 2013/00763-4). P.H.L. Bertolo received a grant from Capes (The Brazilian Federal Agency for Support and Evaluation of Graduate Education). The authors are thankful to the Zoonoses Control Center, Araçatuba (São Paulo, Brazil) for providing the animals for this study.
References
- 1.Saúde Brasil Ministério da. (2014) Manual de vigilância e controle da leishmaniose visceral. Ministério da Saúde. , Brasília
- 2.Baneth G, A F koutinas, Solano-Gallego L, Bourdeau P, Ferrer L. (2008) Canine leishmaniosis: new concepts and insights on an expanding zoonosis, part one. , Trends Parasitol 24, 324-330.
- 3.R A Barata, Silva J C F, R T Costa, Dias C L F, J C Silva. (2004) Phlebotomine sand flies in porteirinha, an area of american visceral leishmaniasis transmission in the state of Minas Gerais. , Brazil. Mem. Inst. Oswaldo Cruz 99, 481-487.
- 4.Dantas-Torres F, S M Latrofa, Otranto D. (2011) Quantification of Leishmania infantum DNA in females, eggs and larvae ofRhipicephalussanguineus. , Parasit. Vectors 4, 56.
- 5.Solano-Gallego L, Miró G, Koutinas A, Cardoso L, M G Pennisi. (2011) LeishVet guidelines for the practical management of canine leishmaniosis. , Parasit. Vectors 4, 86.
- 6.Almeida A B P F, A J Mendonça, Sousa V R F. (2010) Prevalência e epidemiologia da leishmaniose visceral em cães e humanos, na cidade de Cuiabá. , Mato Grosso, Brasil. Cienc. Rural 40, 1610-1615.
- 7.Koutinas A F, Carlotti D N, Koutinas C, E I Papadogiannakis, G K Spanakos. (2010) Claw histopathology and parasitic load in natural cases of canine leishmaniosis associated withLeishmania infantum.Vet. , Dermatol 21, 572-577.
- 8.Figueiredo M M, Deoti B, Amorim I F, Pinto AJW, Moraes A. (2014) Expression of regulatory T cells in jejunum, colon, and cervical and mesenteric lymph nodes of dogs naturally infected withLeishmania infantum.Infect. , Immun 82, 3704-3712.
- 9.Brasil Ministério da Saúde. (2008) Portaria interministerial Nº 1.426, de 11 de julho de 2008-Proíbe o tratamento de leishmaniose visceral canina com produtos de uso humano ou não registrados no Ministério da Agricultura, Pecuária e Abastecimento. Ministério da Saúde. , Brasília 2, pp..
- 10.Tolosa E M C, C J Rodrigues, O A Behmer, A G Freitas-Neto. (2003) Manual de técnicas para histologia normal e patológica. , Manole, São Paulo 331, pp..
- 11.W L Tafuri, R L Santos, Arantes R M E, Gonçalves R, M N. (2004) An alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. , J. Immunol. Methods 292, 17-23.
- 14.D W Scott, W H Miller, C E Griffin. (2001) Claw diseases. In: Muller & Kirk’s (Eds.), Small Animal Dermatology. W.B.Saunders. , Philadelphia 1190-1200.
- 16.A M Hargis, P E Ginn. (2013) O tegumento. Bases da patologia em veterinária. Elsevier, Rio de Janeiro (Eds.) , In: Zachary, J.M., McGavin, M.D 975-1087.
Cited by (1)
- 1.Ramos Fernanda Ramalho, Gouveia Bethânia Almeida, Amâncio Maria Angélica Dias, Carvalho Adolorata Aparecida Bianco de, Vasconcelos Rosemeri de Oliveira, 2024, Comparative study of parasite load in the spleen, lymph node, and skin of dogs with visceral leishmaniasis, Brazilian Journal of Veterinary Pathology, 17(2), 84, 10.24070/bjvp.1983-0246.v17i2p84-92
