Two-Phase Lung Damage Mechanisms For COVID-19 Disease, and Driving Force and Selectivity in Leukecyte Recruitment and Migration
Abstract
To understand lung damages caused by COVID-19, we deduced two phases lung damage mechanisms. After the lungs are infected with COVID-19, the affected lung tissue swells and surface properties of pulmonary capillaries change, both contributing to an increased flow resistance of the capillaries. The initial damages are mainly fluid leakage in a limited number of involved alveoli.
The increased vascular resistance results in retaining more white blood cells (“WBCs”) in pulmonary capillaries. Some of the WBCs may get into interstitial spaces. When more and more WBCs are dynamically retained, the vascular resistance of pulmonary capillaries further rises; and thus the overall vascular resistance of the lungs rises and pulmonary pressure rises. The rise in the pulmonary pressure in turn results in elevated capillary pressures. When pulmonary capillary pressures around the alveoli are sufficiently high, the elevated pressure causes interstitial pressures to change from normally negative values to positive values. The positive pressures cause fluid leakage to the alvoeli and thus degrade lung function. Tissue swelling, and occupation of WBCs in interstitial spaces and occupation of alvoelar spaces by leaked water result in reduced deformable and compressible spaces, and thus causes a further rise of the vascular resistance of the lungs. When the pulmonary pressure has reached a critical point as in the second phase, the blood breaks capillary walls and squeezes through interstitial spaces to reach alveolar spaces, resulting in irreversible lung damages. Among potential influencing factors, the available space in the thorax cage, temperature, and humid are expected to have great impacts. The free space in the thorax cage, lung usable capacity, and other organ usable capacities are the major factors that determine the arrival time of last- phase irreversible damage. The mechanisms imply that the top priority for protecting lungs is maintaining pulmonary micro-circulation and preserving organ functions in the entire disease course while controlling viral reproduction should be stressed in the earliest time possible. The mechanisms also explain how leukecytes are “recruited and migrated” into inflamed tissues by dynamic retention.
Author Contributions
Copyright © 2022 Jianqing Wu, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
The pathological features of lung damages caused by SARS has been described 1. The lungs were edematous and increased in weight with extensive consolidation. The damages include extensive edema, glossy membrane formation, collapse of alveoli, scaling of alveolar epithelial cells, and fibrous tissue in alveolar spaces. The pathological features of damaged lungs of COVID-19 patients have been reported 2. The patient died from a sudden cardiac arrest.
The lungs showed bilateral diffuse alveolar damage with cellular fibromyxoid exudates. The left lung tissue displayed pulmonary oedema with hyaline membrane formation. Interstitial mononuclear inflammatory infiltrates, dominated by lymphocytes, were seen in both lungs. To find best treatments for the COVID- 19 disease, it is essential to understand the mechanisms by which the lungs are damaged by the COVID-19 virus. Yoo et al. have conducted a review on viral infection of lungs and host innate and adaptive responses 3. In that review, they discussed more than twenty types of immune and other cells and their functions in initiation, resolution and restoration phases. Exact functions of many of immune cells were unknown. We are interested in finding lung damage mechanisms that would enable health care givers to understand how the lungs are damaged and what factors control the course of lung damages. Since our purpose is for exploring factors that determine lung damages, we will not include the great details that were known. Existing knowledge could be incorporated into our proposed mechanisms.
Methods
In this theoretical study, we found and used well known data related to the COVID- 19 diseases, lung structure, lung physiology, physiological data, blood composition, viral replication, physical factors, environmental factors, etc to predict micro-circulation condition in lungs, change in blood pressure, and changes in lung and other organs. We conducted several simulations to see how the retention of WBCs at various rates can take up free deformable and compressible volume in the thorax cage. Our suspect is that when the free space is occupied by leaked blood fluid and exudates, the vascular resistance in lungs rapidly goes up and results in heart arrest or irreversible damages to the lungs.
We then propose two-phase mechanisms and use the mechanisms to predict how each of those well known factors affect the course of lung damages.
Results
Two-Phase Lung Damage Mechanisms
White blood cells (“WBCs”) pass through pulmonary capillaries by deforming themselves and squeezing through 4. There is a great size discrepancy between the mean diameter of circulating leukocytes (6-8 µm) and that of the pulmonary capillaries (~5.5 µm). Small lymphocytes are 7 to 10 µm in diameter, and large lymphocytes are approximately 14 to 20 µm in diameter.
Inflammation-Driven Progressive Lung Damage Process
The endothelium actively participates in controlling blood flow, and affect permeability, leukocyte infiltration, and tissue edema 4, 5, 6. The changes in epithelial cells disrupt blood homeostasis by increasing capillaries’ vascular resistance. Other changes include flow dysregulation, thrombosis, and capillary leaks 5. The origin and differentiation cues for many tissue macrophages, monocytes, and dendritic cell subsets remain unclear 7. The finding of retention of leukocytes provides several hints. All white blood cells are produced and derived from multipotent cells in the bone marrow known as hematopoietic stem cells. As long as leukocyte concentration in blood can be detected at certain level, a considerable part of them are predicted to enter the lung tissue and exit from the lung tissue except those that die. We can deduce that the cell retention time depends on not only the hole diameters of capillaries but also the elasticity of the capillaries. Second, it is obvious that the micro-vascular network of capillaries can be blocked by an excessive number of retained of WBCs. Finally, blood viscosity must be an important influencing factor. Thus, all factors that affect the blood viscosity affect vascular resistance and the pulmonary blood pressure.
Figure 1 shows how the retention of WBCs is responsible for damages to the lungs.
Figure 1.The figure shows how large WBCs are retained dynamically when they are moving through pulmonary capillaries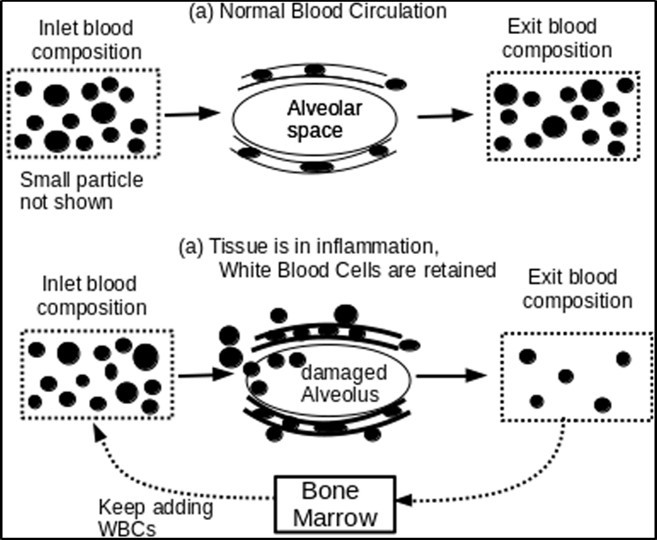
Diagram (a) in Figure 1 shows how WBCs squeeze through capillary network with much smaller pores. Diagram (b) shows that when the tissue is in inflammation, the walls of capillaries are changed and the occupation of WBCs in the interstitial spaces will generate normal force against the walls of capillaries, and thus raise friction against the moving of WBCs. Some large WBCs are retained and thus result in higher local capillary pressure. Initially, water leaks out from a limited number of blood vessels and passes through interstitial spaces to reach the space of nearby alveolus. When local blood pressure rises further to pass a threshold that most capillaries walls can withstand, as in the second phase, the circulating blood forces WBCs to break through capillaries walls and squeeze through the space between epithelial cells to reach the spaces of the alveolus.
Early phase Infection leads to swelling and changes in epithelial cells, which in turn raise the vascular resistance of pulmonary capillaries, raise local capillary pressures and increase interstitial pressures. Normal capillary pressure at a middle point is about 7 mm Hg. If the capillary is blocked in the venous side, the pressure is same as the arterial pressure (about 15 mm Hg mean). This results in an increase in the interstitial pressure. The reversal of the interstitial pressure leads to fluid leakage to the alveoli, and, if the capillary pressure is too high, the blood raptures epithelium of alveoli and reach alveoli inner spaces in a limited number of alveoli. In the early phase, lung injury is caused by damages to a limited number of alveoli as sporadic incidences.
Lungs are a highly expandable and deformable organ. A healthy adult can have 3000 ml inspiration volume while the normal breathing takes about only 500 ml volume 8. This implies that lungs have about 2500 ml extra deformable and compressible space. The air in lungs is compressible, and even the blood is also “compressible” because some of the blood can be squeezed out during compression. If blood flow meets higher flow resistance, the lungs would be less compressible. The lungs are also deformable because the lungs could be deformed to occupy any part of the free space in the thorax cage. Moreover, during breath, the lungs can periodically occupy the space generated by downward moving of the diapharam. When a person does the maximum inspiration, the force drives a considerable amount of blood out of the lungs.
However, if the blood circulation is partially or severely jammed, both water and blood cells are nearly non-compressible. Thus, leaked blood reduces available space for capillaries to expand, and has an equivalent effect of reducing capillary deformability or elasticity. Affected tissue has an increased vascular resistance to blood circulation, which further promotes the retention of WBCs at higher rates.
If the inflammation is of a limited degree, the slower traveling speed of WBCs has an effect of extending the WBCs’ dwell times so that they can have more time to contact infected cells and foreign matters. However, on a long term basis, the body must maintain balance that the number of entering WBCs must substantially be equal to the number of exiting WBCs. We refer this requirement as WBCs transport balance for convenience. This balance is absolutely vital and determine lungs health and the host person’s life.
COVID-19 infection or other lungs infection disturbs the normal WBCs transport balance. As a result, some WBCs may stay in the capillary for too long while certain large WBCs may being caught indefinitely. The infected tissue keeps retaining WBCs. By perpetual accumulative effects, the occupation of WBCs in interstitial spaces and slow-travel of WBCs in capillary pores result in higher vascular resistance. The retention of WBCs results in a reduction of WBC concentration in blood. A reduction of the WBCs concentration in the blood causes bone marrows to generate more WBCs 3, 5.
When newly arrived WBCs travel through the lungs, they are again caught and retained dynamically. Eventually, accumulated WBCs occupy too much of interstitial spaces, and leaked fluid and blood exudates fill more alveolar spaces. The pulmonary vascular resistance reaches the maximum and shuts down pulmonary circulation as heart arrest or multiple organs failure. The most obvious damages are found on alveoli. Alveoli are filled with viscous materials and WBCs 2, 3.
Healthy lungs are highly elastic and have ample room for alveoli and capillaries to expand during breathing cycles. While the WBCs are accumulated in interstitial spaces and alveolar air spaces, blood circulation in affected locations becomes worse and worse. In the affected locations, normal blood circulation is increasingly replaced by extremely-slow diffusion process. As a result, some lung cells die from lack of energy and oxygen. To replace dead tissues, lungs generate fibroblastic cells.
The total volume of compressible alveolar spaces is estimated to be 2000- 3000 mL. Since part of this compressible space is attributed to reduced lung blood volume, we use 2000 ml. The heart of an adult person pumps blood at 5 liters per min, The pulmonary flow is essentially same as the cardiac output. WBCs make up approximately 1% of the total blood volume. Assuming that only 0.1%of the WBCs are retained for any time increment, the retention rate would be equivalent to 0.05 ml volume of WBCs per minute. The retention of WBCs in interstitial spaces has the same effect of reducing the volume of alveolar spaces because the total volume of the lungs is substantially fixed. The fluid in alvoelar spaces is not compressible. Free volume occupied by retained WBCs are shown in Table 1.
The filled volume can also be estimated by computing the WBC volume. In a normal adult, there are 4.3-10.8 ⨯10(9) WBCs per liter of blood. Assuming 0.1% of the largest WBCs are retained in any given time, we got a similar trend (Table 2).
Table 1. Percent of Lung Compressible Space Token By Blood Exudate Increases with Time (Based on 0.1% WBCs retention volume)| Retention Vol rate.(ml/min) | Time (min) | Time (various) | Exudate Vol. (ml) | Percent of CompressibleVol. (%) |
| 0.05 | 1 | 1 min | 0.05 | 0.0025 |
| 0.05 | 60 | 1 hour | 3 | 0.15 |
| 0.05 | 1440 | 1 day | 72 | 3.6 |
| 0.05 | 7200 | 5 days | 360 | 18 |
| 0.05 | 14400 | 10 days | 720 | 36 |
| 0.05 | 28800 | 20 days | 1440 | 72 |
| 0.05 | 43200 | 30 days | 2160 | Over-limited |
| WBC No | Each Cell Vol.(cu.µm) | Vol. Retention Rate (mL/min) | Time (min) | Time (various) | Exudate Vol. (ml) | Percent of Lung Compressible Vol. (%) |
| 4E+07 | 1000 | 0.035 | 1 | 1 min | 0.035 | 0.0018 |
| 4E+07 | 1000 | 0.035 | 60 | 1 hour | 2.1 | 0.105 |
| 4E+07 | 1000 | 0.035 | 1440 | 1 day | 50.4 | 2.52 |
| 4E+07 | 1000 | 0.035 | 7200 | 5 days | 252 | 12.6 |
| 4E+07 | 1000 | 0.035 | 14400 | 10 days | 504 | 25.2 |
| 4E+07 | 1000 | 0.035 | 28800 | 20 days | 1008 | 50.4 |
| 4E+07 | 1000 | 0.035 | 43200 | 30 days | 1512 | 75.6 |
The discrepancy between the two methods may be attributed to the approximate volume of WBC cells and estimated mean WBC cell volume. The exact numbers are not important because all of those parameters can vary considerable anyway. What is important is that WBCs retention is a parameter that can control the lung performance and the volume token by retained WBCs can progressively impair the lungs in a time window similar to observed disease time window. In those computations, the lungs have considerable compressible space (for a healthy person). The situation would be much worse for people who even experience shortness of breath in their daily lives.
When the lungs cannot maintain WBCs transport balance, the lungs may fail within five to ten days. If the retention rate of WBCs increases to 1%, the patient may die in one to two days. This happens when a big part of alveolar spaces are filled by extruded blood and leaked fluid.
Critical Point of Irreversible Lung Damages
We found there is a critical point for the lungs to experience irreversible damage. The systolic pulmonary pressure is about 25 mm Hg and diastolic pulmonary pressure is about 8 mm Hg, with the mean pulmonary arterial pressure being about 15 mm Hg. The negative pressures in interstitial spaces is maintained by the flow caused by lymphatic pumping, and net osmotic pressure. Extra fluid that has been on alveoli is sucked back to the lung interstitium through the small openings between epithelial cells. Damage to the capillary membrane causes leakage of fluid and plasma proteins and thus result in an increase in the interstitial pressure. The edema of the interstitium results in a raised interstitial pressure, which can cause immediate rapture of the epithelium.
When a sufficient number of capillaries are “blocked” by slow-moving or retained WBCs, the overall vascular resistance rises; and slow-moving WBCs in capillaries reduce the “expandable” volume of the blood vessels in the lungs. An elevated pulmonary pressure in turn raises capillary pressures for all alvoeli. The interstitial pressures are directly related to capillary pressures, and become positive when venous pressure is elevated 8, 9. If the capillary pressure around an alvoelus is sufficiently high, the outward pressure will be larger than inward pressure. There must be a point at which the pressure at the interstitial space is changed from the normal negative value to a positive value. It is inferred that after a sufficient number of WBCs has been retained, it has a global impact on the lungs. For this reason, infection of a sufficient large number of alveoli can cause damages to substantially all alveoli through raising the pulmonary pressure.
Potentially Doubly Exponential Damaging Curve
In the above computations, we did not consider two self-aggravating factors. We predicted that lung function degrades potentially by a doubly exponential curve for the following reasons. First, retained WBCs and lung swelling are expected to make pulmonary vascular circulation progressively worse. The expected failure to maintain energy metabolism further aggravates inflammation and diminishes the heart ability to maintain required pulmonary vascular circulation. Thus, the speed of lung damage at a later time intervals is faster than that at previous time intervals. Moreover, the lungs have a fixed total volume and all expandable spaces including the “compressible” volume of blood vessels are required for normal breathing. When some compressible spaces are filled by incompressible fluid and WBCs, their adverse impacts cannot be linear. There is a point at which BWCs cannot pass through.
When more of the lung voids are filled by fluid and WBCs, the pulmonary vascular resistance rises rapidly. The elevated pulmonary pressure forces blood to squeeze into and through any spaces in the entire lungs. It may take a short time, possibly in a matter of less than an hour to complete the final stage of irreversible damages. When substantially all elastic spaces are occupied by WBCs and fluid, the pulmonary vascular resistance approaches the maximum, pulmonary flow reaches zero, and lung function approaches zero. There is no way to stop or reverse.
Considering the potentially doubly exponential damaging process, we estimate that dynamic retention rates of WBCs could be 0.01%-0.1% initially, increase to 0.1% to 1% when the lungs lose most function; the blood rapidly fills the voids in the lungs finally. Our ballpark prediction is consistent with the rapid disease course from shortness of breath to death 3. The damaging process implies that the problem is correctable by using right methods only in the earliest time. We cannot over stress the importance of this strategy.
Physiological Injury of Low Temperature to the Lungs
Low temperature is known to affect flu 14 and blood vessels 13. Low temperature causes capillaries to contract to add more friction to WBCs traveling and cause some WBCs retained indefinitely. Low temperature promotes fluid leakage to the affected alveolus and hinders oxygen-carbon dioxide exchange. Reduced oxygen delivery causes blood vessel vasodilation 23 and make the situation worse. Low temperature also adversely affects the lungs by influencing blood viscosity. For a segment of capillary, its vascular resistance can be determined by equation R = 8ηl/πr4. Low temperature affects the flow resistance by the viscosity term and the radium to the fourth power (r4). This is why exposure to low temperature is the biggest aggravation factor of cold, flu and COVID-19. If a large number of WBCs is in the blood, they raise local vascular resistance. Low temperature might dramatically impact the travel-through of big WBCs. If one or more WBCs are retained in the capillary or move too slowly along the capillary pore, the bulky fluid of blood has to squeeze though the tiny void between the surface of retained WBCs and the capillary wall. Low temperature can have a big role in causing interstitial pressures to change from negative to positive and blood leakage into the alveoli. Low temperature may cause the critical time of damage to arrive earlier.
Since the mechanisms tell only how the lungs are damaged reversibly or irreversibly, such mechanisms are not enough for predicting the severity of lung damages or death. Thus, we must focus on lung structures and personal health. It is well known that a person’s ability to survive depends on their vital functional reserves 10, 11, 12. Those functions provide additional useful information about a person’s ability to resolve infection. Many other factors can aggravate damages to alveoli by influencing mobility of WBCs. They include red blood cells count, platelets aggregation degree, and natures and amounts of other macro-molecules because they can affect blood viscosity. Moreover, chronic stress and emotional distress on disease outcomes 16, 17, 18, 19, 20, 21, 22 (to be explained later). Free space in the thorax cage and body mechanic vibrations are two unique factors.
Further Lung Damages Induced by Insufficient Lung Function
If the lungs are unable to perform required functions, expected degraded energy metabolism leads to a diminished lung function and leads to failure of major organs such as heart, kidneys, and liver. Those processes are shown in Figure 2.
Figure 2.It Shows how the virus-triggered WBCs retention can impair other vital organs such as liver, heart and kidneys, resulting in final heart failure or multiple organ failure.
Figure 2 shows how viral infection triggers the retention of WBCs and causes damages to alveoli as indicated by the round circular diagram. The damages to alveoli results in higher pulmonary vascular resistance and degraded energy metabolism. The degraded energy metabolism will impair heart, kidneys, liver, etc. The increased vascular resistance and impaired heart, renal and liver function inevitably result in heart failure (as indicated by dashed red arrows).
The human ability to survive depends on vital usable functional capacities of heart, kidneys and liver 10, 11, 12. Virus-caused inflammation diminishes lung functions 3, 4, and causes the lungs to fail to deliver required oxygen for the body. The insufficiency of oxygen must lead to diminished energy production for the whole body. This is expected to cause heart failure 3, 4 and impair renal function 24, 25. The impaired renal function in turn adversely affects the heart 25, 26 and the lungs 27, 28. While only a few references are cited, we confidently found that impairment of any vital organ must finally result in failure of heart or multiple organ failure as long as the impairment of the vital organ is sufficiently long. By going through those vicious cycles, the viral infection has an effect of retaining more WBCs in the tissue, retaining more metabolic wastes, and causing more damages to the lungs, the heart and the kidneys. It is possible that some patients die from organ failure caused by the vicious cycles even before the lungs have reached the critical point of breaking blood vessels if the patient’s organ functions in the heart or other vital organs play determinant roles.
Severe lung damages could be caused by viral damages before the start of adaptive immune responses. This may happen because low temperature causes blood vessels and capillaries to constrict and raises blood viscosity 13. The proposed mechanisms can also explain the effect of humidity on the disease 14. When air humidity is high, water molecules coming from alveolar space are not brought out efficiently. The water layers on alveolar walls is expected to interfere with oxygen-carbon dioxide exchange. The mechanisms can explain the role of blood viscosity, mechanical vibrations, etc. Objects jammed at a bottleneck of a bag can be facilitated by making mechanical vibrations. The mechanisms also explain why old people are more vulnerable to the virus. Old people have diminished organ capacities 10, 11, 12 and their blood vessels are less elastic. The mechanisms also explain the role of chronic stress and emotional distress on disease outcomes 16, 17, 18, 19, 20, 21, 22. When the patient is in a relaxed state, the pulmonary vascular circulation is improved and WBCs encounter less friction.
Driving Force and Selectivity in Leukocyte Recruitment and Migration
Our proposed lung damage mechanisms add more variables to classic leukocytes recruitment theory. Unlike motility in bacterial chemotaxis, mechanism by which leukocytes physically move is unclear. T and B cell homing and transendothelial migration have been extensively studied 29. How neutrophils get activated in a proper way and degree so that they can adhere to the endothelial surface, locomote to right localities and squeeze through small pores is not fully understood 30. Further, no directional signals have been found to cause leukocytes to move to the inflammation site 29. The existing leukocyte recruitment theory can explain that an inflamed tissue selectively retains leukocytes in great details, but could not explain why blood exudates are found in alevoelar spaces.
It is believed that leukocytes take the “path of least resistance” across the endothelium 31. That means that leukocyte migration path through the intercellular space or through cells may depend on the relative tightness of the endothelial junctions and the ability of the leukocytes to breach them. Existing theories do not use local blood pressures as the driving force. We found that elevated blood pressure or pressure gradient, and structural strength in capillaries or interstitial spaces are determining factors.
Some studies have investigated hydrodynamic properties for leukocytes migration. Models they used involve cancer cell culture media 32, adhesive rolling of deformable leukocytes over a coated surface in parabolic shear flow in microchannels 33 or a simple hydrodynamic model 34. Those models do not mimic the structures of lungs and do not consider blood pressures in capillary networks in the lungs. One study implies that cell deformability significantly reduces the flow resistance and that high cell concentration must increase the flow resistance 33.
Erratic and uncontrolled leukocyte migration and accumulation were seen in diseased tissues such as atherosclerotic plaque 35 and rheumatoid arthritic tissue 36. Anti-inflammatory drugs have been used to reduce leukocyte recruitment 37. Those findings as well as personal observations all show that WBCs are accumulated on a tissue that is inflamed. The whole body tissue is like a big filter from which the blood passes through, and WBCs pass through the filter in a steady state condition. Whenever any specific part of the tissue is inflamed, this part of the tissue will selectively retain WBCs by increasing flow friction. This may be intended by the evolution design to increase dwell times for WBCs to perform their functions at the inflamed site. Leukocytes horizontal migration may be limited to diffusion and their preference to move through the path with the lowest resistance. When blood keeps feeding the WBCs into tissues, more of the WBCs reach and stay wherever inflammation has happened. There is no need to recruit WBCs from neighbor tissues in a direction perpendicular to blood flow direction. This may give an impression that WBCs can be recruited in horizontal directions. However, the excessive retention of WBCs becomes a fatal problem if they occupy too much volume in the thorax cage.
Implications of the Lung Damage Mechanisms
Maintaining pulmonary vascular circulation is the top priority in the entire disease course for COVID-19 as well as other lung infection. Maintaining the mobility of WBCs is vitally important to both innate immunity and acquired immune response 39, 40, 41.
Our mechanisms imply that temperature is very important factor. Temperature may regulate immunity by multiple ways 42. Hyperthermic temperatures affect function of all types of cells include DCs, macrophages, NK cells, neutrophils, T and B lymphocytes, and vascular endothelial cells. High temperatures (42°C for 15 min) has been found to blunt leukocyte adhesion even 2 days after the heat treatment in vivo 43. This finding implies that a pre-treatment with warm temperature could mitigate severity of COVID-19 disease that is caused by a subsequent exposure. It is believed that fever temperatures can broadly promote immune surveillance during challenge by invading pathogens 42. Body temperature is controlled by substance interleukin-1 (or leukocyte pyrogen) in the hypothalamus of the brain. Interleukin-1 is released from blood leukocytes and tissue macrophages that have digested viruses and bacteria 8. Raising temperature can improve both immune functions and mitigate lung damages caused by immune cell congestion.
Our mechanisms imply that raising body temperature can help improve pulmonary micro-circulation and keep WBCs transport balance. Patients should be advised to avoid exposure to low temperature and high humidity in the entire disease course. Other measures should be taken to reduce blood viscosity. We question the measure of using drugs to lower body temperature as the standard of care simply because patients demand comfort. Excessive fever can cause damage to the Central Nervous System. A better strategy is maintaining the body at a higher temperature but lowering the head’s temperature by using a cooling bath only if necessary. If cooling is necessary, it should be used only to the extent to avoid fever damages to the brain, but should never overdo.
The mechanisms also imply that antiviral drugs or alternative measures should be taken as early as possible. When the virus has infected the whole lungs and the patient’s lung function has approached a disability level, such a drug treatment may burden the lungs by its side effects. A sound strategy is to reduce tissue inflammation 37, reduce vascular resistance, keep waste removal balance and strengthen vital organs.
The mechanisms imply that age, obesity, and organ usable functions are the most important factors. Age is related to organ reserve 10, 11, 12. However, organ functional reserve may include functions that are not presently useful. We use organ usable function capacity to stress that functional capacities that are actually deliverable. When a person’s lungs have little surplus capacity, the infection can more likely cause the lungs to enter the self-degrading cycle. Obesity must be a critical factor because extra fat tissue consumes too much of the free thoracic space. The severity of obesity is indicated by a large size of abdomen. However, extra tissues inside thorax cannot grow out in the thorax cage because all ribs are not deformable. This implies that some extra tissues can only grow within inner thorax cage, and this is why shortness of breathe is a common sign of obesity. Reduced free space in the thorax cage means that an infection can more quickly raise the pulmonary blood pressure, and such a person has much shorter time to resolve the infection before the lungs get into vicious aggravating stage. In addition, extra tissues in obese persons necessarily increase the demand for all vital organ functional capacities, and reduce body’s ability to clear up metabolic by-products. Like low temperature, obesity adversely affects the patient’s ability to fight COVID-19 disease in multiple ways. That is why losing extra body weight is the most effective strategy to increase chances to survive from the pandemic.
Medicine should explore safe drugs that can dilate blood vessels that can be used to relieve WBC retention.
Funding Statement
The author(s) declared that no grant was used in support of this research project.
Article History
This subject of this article was initially disclosed in part in preprint.org on 6 February 2020, and was later rewritten and posted in substantially current form on preprints.org server on 8 September 2020.
References
- 1.Gu J, Korteweg C. (2007) Pathology and Pathogenesis of Severe Acute Respiratory Syndrome. , The American Journal of Pathology 170, 4.
- 2.Zhe Xu, Shi L, Wang Y. (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine.
- 3.Yoo J-K, Kim T S, Hufford M M, Braciale T J.Viral infection of the lung: Host response and sequelae. , J Allergy Clin Immunol 132(6).
- 4.Downey G P, Doherty D E, Schwab B. (1990) Retention of leukocytes in capillaries: role of cell size and deformability. , J Appl Physiol 69, 1767-1778.
- 5.Pober J S, Sessa W C. (2015) Inflammation and the Blood Microvascular System. Cold Spring Harb Perspect Biol. 7, 016345.
- 6.Mercer B A, Lemaître V, Powell C A, D’Armiento J. (2006) The Epithelial Cell in Lung Health and Emphysema Pathogenesis. Curr Respir Med Rev. 2(2), 101-142.
- 7.Geissmann F, Manz M G, Jung S. (2010) Development of monocytes, macrophages and dendritic cell. Science. 327(5966), 656-661.
- 8.Guyton A C.The cough reflex. In Text of Medical Physiology (8th Ed). W.B. Saunders Company. pg 411-412 (various page rages)..
- 9. (1965) Pressure-volume relationships in the interstitial spaces. Investigative Ophthalmology. Available at iovs.arvojournals.org on 03/01/2020
- 10.WMT Bortz, Bortz W M. (1996) How fast do we age? Exercise performance over time as a biomarker. , J Gerontol A Biol Sci Med Sci 51, 223-5.
- 11.Goldspink D F. (2005) Ageing and activity: Their effects on the functional reserve capacities of the heart and vascular smooth and skeletal muscles. Ergonomics. 48, 1334-51.
- 12.Sehl M E, Yates F E. (2001) Kinetics of human aging: I. Rates of senescence between ages 30 and 70 years in healthy people. , J Gerontol A Biol Sci Med Sci 56(1), 98-208.
- 13.Shepherd J T, Rusch N J, Vanhoutte P M. (1983) Effect of cold on the blood vessel wall. Gen Pharmacol. 14(1), 61-4.
- 14.Anice C Lowen, Steel John. (2014) Roles of Humidity and Temperature in Shaping Influenza Seasonality. Journal of Virology. 88(14), 7692-7695.
- 15.Hawryluck L, Gold W L, Robinson S. (2004) Emerg Infect Dis. SARS Control and Psychological Effects of Quarantine , Toronto, Canada 10(7), 1206-12.
- 16.Steptoe A, Hamer M, Chida Y. (2007) The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. , Brain Behav Immun 21(7), 901-12.
- 17.Segerstrom S C, Miller G E. (2004) Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. , Psychol Bull 130(4), 601-30.
- 18.Dhabhar F S. (2014) Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 58(3), 193-210.
- 19.Walburn J, Vedhara K, Hankins M. (2009) Psychological stress and wound healing in humans: a systematic review and meta-analysis. , J Psychosom Res 67(3), 253-71.
- 20.Webster Marketon JI, Glaser R. (2008) Stress hormones and immune function. Cell Immunol.252(1-2):. 16-26.
- 21.Pedersen A F, Zachariae R, Bovbjerg D H. (2009) Psychological stress and antibody response to influenza vaccination: a meta-analysis. Brain Behav Immun. 23(4), 427-33.
- 22.Pedersen A, Zachariae R, Bovbjerg D H. (2010) Influence of psychological stress on up- per respiratory infection—a meta-analysis of prospective studies. Psychosom Med. 72(8), 823-32.
- 23.Michiels C. (2004) Physiological and Pathological Responses to Hypoxia. , The American Journal of Pathology 164(6), 1875-82.
- 24.Chihanga T, Ruby H N, Ma Q. (2018) NMR-based urine metabolic profiling and immunohistochemistry analysis of nephron changes in a mouse model of hypoxia-induced acute kidney injury. , Am J Physiol Renal Physiol 315(4), 1159-1173.
- 25.YM1 Arabi, Al-Omari A, Mandourah Y. (2017) Critically Ill Patients With the Middle East Respiratory Syndrome: A Multicenter Retrospective Cohort Study. Crit Care Med. 45(10), 1683-1695.
- 26.Ter Maaten JM, Damman K, Verhaar M C. (2016) Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. , Eur 18(6), 588-98.
- 27.Visconti L, Santoro D, Cernaro V. (2016) Kidney-lung connections in acute and chronic diseases: current perspectives. J Nephrol. 29(3), 341-348.
- 28.Domenech P, Perez T, Saldarini A. (2017) Kidney-lung pathophysiological crosstalk: its characteristics and importance. Int Urol Nephrol. 49(7), 1211-1215.
- 29.Muller W A. (2016) Transendothelial Migration: Unifying Principles from the Endothelial Perspective. Immunol Rev. 273(1), 61-75.
- 30.Heemskerk N. (2016) F-actin-rich contractile endothelial pores prevent vascular leakage during leukocyte diapedesis through local RhoA signalling. , Nat Commun 7, 10493.
- 31.Carman C V, Springer T A. (2008) Trans-cellular migration: cell-cell contacts get intimate. , Curr Opin Cell Biol 20, 533-540.
- 32.NNO Ngalame, Luz A L, Makia N, Tokar E J. (2018) Arsenic Alters Exosome Quantity and Cargo to Mediate Stem Cell Recruitment Into a Cancer Stem Cell-Like Phenotype. Toxicological Sciences. 165(1), 40-49.
- 33.Pappu V, Doddi S K, Bagchi P. (2008) A computational study of leukocyte adhesion and its effect on flow pattern in microvessels. J Theor Biol. 254(2), 483-98.
- 34.Subramaniam D R, Gee D J. (2018) The influence of adherent cell morphology on hydrodynamic recruitment of leukocytes. , Microvasc Res 115, 68-74.
- 35.Li J, Ley K. () Lymphocyte migration into atherosclerotic plaque. Arteriosclerosis, Thrombosis, and Vascular Biology. 35(1), 40-9.
- 36.Rana A K, Li Y, Dang Q, Yang F. (2018) Monocytes in rheumatoid arthritis: Circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis. , International Immunopharmacology 65, 348-359.
- 37.Planagumà A, Domènech T, Pont M. (2015) Combined anti CXC receptors 1 and 2 therapy is a promising anti-inflammatory treatment for respiratory diseases by reducing neutrophil migration and activation. , Pulmonary Pharmacology & Therapeutics 34, 37-45.
- 38.Westphalen K, Gusarova G A, Islam M N.Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. , Nature 506(7489), 503-6.
- 39.Kirby A C, Coles M C, Kaye P M.2009;Alveolar macrophages transport pathogens to lung draining lymph nodes. , J Immunol 183(3), 1983-9.
- 40.Bhattacharya J, Westphalen K. (2016) Macrophage-epithelial interactions in pulmonary alveoli. Semin Immunopathol. 38(4), 461-469.
- 41.Rodero M P, Poupel L, Loyher P-L. (2015) Immune surveillance of the lung by migrating tissue monocytes. eLife 4:. 07847.
Cited by (3)
This article has been cited by 3 scholarly works according to:
Citing Articles:
Natural Product Research (2025) Crossref
Global Journal of Cancer Therapy (2023) OpenAlex
Global Journal of Cancer Therapy (2023) Crossref