Therapeutic Potential of Autologous Adipose Derived Mesenchymal Stem Cells in Human POI and Ovarian Aging
Abstract
Background
Women play an important role in the work setting. This leads them to put off their motherhood, sometimes preventing them from getting pregnant. Delaying pregnancy face women with low ovarian response, such as in Premature Ovarian Insufficiency (POI) or Ovarian Aging (OA). There is no current treatment, although there is evidence of improving ovarian function by inyecting mesenchymal stem cells (MSC).
Materials and Methods
Prospective, observational study of 17 women who attended Pronatal Clinic from 2019 to 2020. Each patient was registered in Assisted Reproductive Treatment (ART) and was enrolled in ovarian treatment with an autologous adipose tissue Mesenchymal Stem Cell (AD-MSCs) protocol. Three groups were assembled: 1) Control: AMH >1.2 ng/mL, without AD-MSCs, 2) POI/OA: female infertility due to POI/OA with AMH <1.2 ng/mL and 3) Amenorrhea: female infertility due to POI/OA with amenorrhea and AMH <1.2 ng/mL. Variables: Age, weight, height, serum AMH, endometrial thickness, follicular size and number on day 2 and 11 of the menstrual cycle, oocyte number, number of blastocysts and pregnancy rate.
Results
Between month 2 and 5, after AD-MSCs inyection, POI/OA group showed an increase in follicle number (2 to 9) and size (13.5 to 15.5 mm) on day 11 of the menstrual cycle, which resulted in a higher number of MII oocytes (2.6 to 4.2), and an increase in number of blastocysts (0 to 3) and endometrial thickness (8.6 to 9.4). Regarding the Amenorrhea group, a reboot in menstrual cycle was observed, although no further development of blastocyst was found.
Conclusion
The AD-MSCs inyection directly in the ovary allowed an increase in number of blastocysts and improved pregnancy rates in POI/OA patients.
Author Contributions
Academic Editor: Ang-Chen Tsai, PBS Biotech, United States
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2021 Luján Irastorza Jesús Estuardo, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
In Mexico, 17% of women of reproductive age have infertility issues, which results in 1.4 million of couples in need of assisted reproductive techniques 1, 2. From 9 to 25% of these women may show a poor ovarian response, which is defined as a low oocyte collection after an ovarian stimulation 3, 4. This condition might be associated to Premature Ovarian Insufficiency (POI) characterized by an increment in Follicle Stimulant Hormone (FSH) and Luteinizing Hormone (LH) levels, with a reduction in Anti-Müllerian Hormone (AMH) and estrogen levels, including menstrual modifications before the age of 40 (oligomenorrhea or amenorrhea) 5, 6, 7. This condition has a prevalence of 1 out of 100 women before the age of 40; and in 1 out of 1000 under 30, and the risk variation depends on race, which ranges from 0.1% in Japanese to 1% in Caucasians, and 1.4% in Africans and Hispanics 5, 6, 7. Several POI etiopathogeneses have been described; i.e., autoimmune diseases 8, oxidative stress 9, genetical predisposition 10, radiotherapy and chemotherapy in the treatment of cancer 11, 12, 13. All of these may cause problems in the collection and maintenance of primordial follicles in addition to follicular atresia and apoptosis of granulosa cells 14, 15, 16, 17. Only 5 to 15% of these patients are able to get a spontaneous pregnancy 18, 19, 20.
In addition to the above, currently, women have an important roll in the labor sector, leading them many times to delay motherhood up to an age where their fertility has diminished so much that prevents them to get pregnant. This is the consequence of a reduction in the number of follicles, which are lost every period. As is well known, women are born with a mean of 1 to 2 million of primordial follicles, which decrease to 500,000 in puberty; about 1000 are lost per month after menarche, and after age of 35, this rate get higher 21, 22, 23, 24, 25. This constitutes the beginning of a reduction in ovarian function characterized by an increase in gonadotrophin levels (LH and FSH), and a decrease in AMH and estrogen levels until menstruation finally stops, phenomenon known as menopause, as a consequence of the low number of follicles, which results in an inability to maintain the menstrual cycle 25, 24, 26, 27.
In POI (premature aging) as well as in natural Ovarian Aging (OA), there are many instances where the only option that patients have to get pregnant is to accept an ovule donation 28, 29. This situation is not welcome by some couples and the lack of treatment for restoring ovarian function have lead some specialists to investigate new treatment alternatives; i.e., mesenchymal stem cells (MSCs).
MSCs are found in embryonic and adult tissue. In both of the cases these cells have the ability of auto-renovation, they show a high proliferation and differentiation rate and the ability to differentiate to different cell lines (multipotent) 30, 31, 32, 33. MSCs may be obtained from bone marrow tissue (BM-MSCs) 34, peripheral blood (PB-MSCs) 35, umbilical cord (UC.MSCs) 36, adipose tissue (AD-MSCs) 37, menstrual blood (Men-MSCs) 38, etc. They also may be autologous (self cells) or allogenic (donator cells) cells 39, 40, 41. Mesenchymal stem cells have been observed to be capable to produce and release growth factors, cytokines, chemokines, enzymes, etc., which induce a paracrine regulation of anti-inflammatory processes, the generation of new blood vessels (angiogenesis), inhibition of apoptosis, and tissular fibrosis 33, 42, 43, 44, 45, 46, 47. In addition, there are observations in vitro that MSCs grown under suitable conditions are capable to differentiate into cells from the three embryonic germ layers (endoderm, mesoderm, and ectoderm) 47, 48. The term “mesenchymal“ was coined by Caplan in 1991, because of their ability to differentiate into multiple cell types from connective tissue, in addition to their mesodermal origin 49.
All of these features make MSCs very attractive to be used in medicine as shown by studies carried out in laboratory animals and humans, where some recovery in diseases and pathologies as Alzheimer 50, lateral amyotrophic sclerosis 51, Huntington disease 52, Parkinson disease 53, brain and myocardial infarctions 54, 55, immune conditions 56, arthrosis 57, restoring of ovarian function 2, 58, etc., has been observed.
Considering all of the above, the objective of this study is to describe the effects that autologous AD-MSCs have on the ovarian reserve and the obtention of blastocytes, in couples that have not conceived successfully after several attempts by diverse assisted reproductive techniques; couples to which an AD-MSCs therapy was offered as an aid to improve their fertility.
Materials and Methods
This is a prospective and observational study of 17 women that attended to PRONATAL Clinic (Bité Médica Hospital) between 2019 and 2020. Patients were included in protocols of assisted reproductive techniques (ART) and they were proposed to be included in the protocol of ovarian therapy using autologous AD-MSCs as an adjuvant.
Patients were explained about the nature of the proceeding to be carried out and they accepted AD-MSC therapy as an aid after signing a written informed consent. Additionally, the obtention, handling, and transportation of stem cells collected from adipose tissue was made according to the provisions of the Mexican Official Standard NOM-012-SSA3-2012 (that establishes the Criteria for the Realization of Health Research Projects in Human Beings), NOM-260-SSA1-2015 (Mexican Official Standard Project (10/5/17) about disposition of Stem Cells and Progenitor Cells for Therapeutic and Research Purposes); the provisions of Cofepris (Federal Commission for Protection against Sanitary Hazards), UN recommendations, and international regulations.
All cases were evaluated by means of medical history, laboratory studies (general condition, liver, kidneys, thyroid gland, coagulation profiles, ovarian function (AMH) gynecologically, and by ultrasound (Philips, Affiniti 50, with at least 7 MHz)). Inclusion criteria (patients included in the AD-MSCs protocol): Candidates for AD-MSCs procurement, women with normal karyotype, women of reproductive age, infertility caused by POI or OA (menopause), FSH ≥ 20 IU/mL, women with amenorrhea that showed at least one follicle on day 2 or 11 of the menstrual cycle, AMH < 1.2 ng/mL, patients coming back for examination between the second and sixth month after application of AD-MSCs. Additionally, the control group included patients with AMH ≥ 1.2 ng/mL that were not included in this protocol.
Exclusion Criteria (patients excluded from the AD-MSCs protocol): women with autoimmune diseases, lupus erythematosus, multiple sclerosis, coagulation conditions, breast and ovary cancer, contradictions to get pregnant, and lactating women.
Parameters to be Evaluated
Age, weight, height, AMH, ET (endometrial thickness), F2 y F11 (follicles observed on day 2 and 11 of the menstrual cycle), FS2 y FS11 (Follicle Size observed on day 2 and 11 of the menstrual cycle), NO (Number of Oocytes obtained at stage MII, MI and germ vesicles), MII (viable oocytes), FO (total Fertilized Oocytes after evaluation at 18 h), PET (Patients with Embryo Transfer), NB (Number of viable Blastocysts on day 5), PRET (Pregnancy Rate of patients with only Embryo Transfer), PAP (Pregnancy rate for All Patients), and MCR (Menstrual Cycle Recovery).
Patients were divided in 3 groups:
1. CONTROL: AMH>1.2ng/mL, with no procuration of AD-MSCs.
2. POI/OA: women with infertility by POI or OA with AMH <1.2 ng/mL.
3. AMENORRHEA: women with infertility by POI or OA with amenorrhea and AMH <1.2 ng/mL.
Statistical Analysis
Age, weigh, height, AMH, ET, F2, F11, FS2, FS11, NO, MII, FO, PET, and NB were reported as the mean (X̅) ± standard error (SE). On the other hand, the difference among incidence in the diverse groups was determined by a Student’s t test with the help of the statistics software SPSS ver. 25.
Procuration and Preparation of AD-MSCs
On day 14th of menstrual cycle, the patient was brought to the operating room, where adipose tissue was obtained by selecting the site, followed by antisepsis and anesthesia. Sixty cubical centimeters of solid fat were obtained from which 40x106 AD-MSCs were extracted, diluted in platelet-rich plasma (PRP) until a volume of 8 mL was reached. Immediately after the obtention of AD-MSCs, the patient was placed in lithotomy position before general anesthesia was given. Ovaries were located by vaginal ultrasound (Philips, Affiniti 50, with at least 7 MHz). Once located the ovaries, the collection of AD-MSCs was carried out with a follicular aspiration needle. Each ovary was punctured twice and each puncture introduced 2 mL of PRP with 10x106 AD-MSCs. In total, 20x106 AD-MSCs were applied in each ovary (Figure 1).
Figure 1.Technique; Application of PRP + AD-MSCs in the ovary; 1) The aspiration system is closed to prevent PRP + AD-MSCs from going to the oocyte collection tube; 2) PRP + AD-MSCs were injected with the flow of the system directed to the ovary, and 3) It is observed whether PRP + AD-MSCs are deposited in the ovary.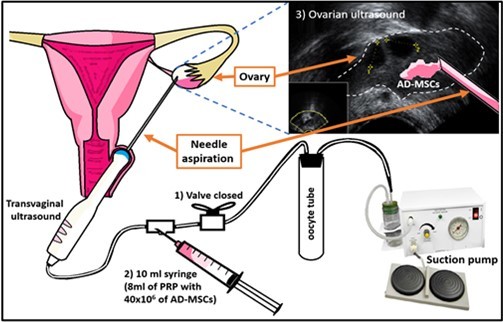
Patient Follow Up
Patients were given an appointment by the beginning of the second cycle after AD-MSCs procuration; on Day 2 the number and size of follicles was evaluated, as well as the presence of menstruation. On Day 3 ovarian stimulation begun with 300 IU of hMG (Merapur®) per day from Day 3 to Day 11, and 0.25 mg per day of cetrorelix (Cetrotide) from Day 8 to Day 11, and on Day 11 hCG (Ovidrel) was applied, and the number of follicles and endometrium thickness were evaluated. Afterwards, on Day 14 a follicular aspiration was carried out and the total number of oocytes obtained was estimated, in addition to MII in a separated way. After follicular aspiration, MII oocytes in were fertilized and the number of fertilized oocytes 18 h after fertilization was evaluated, as well as the number of Day 5 blastocytes obtained. Finally, the patients successful in the obtention of blastocytes were subjected to an embryo transfer (PTET and PAP evaluated). Patients with a low number and small sized follicles received an appointment for the following menstrual cycle, for examination and to evaluate whether an improvement was observed or not, and then to begin ovarian stimulation (this procedure was repeated until the sixth examination or menstrual cycle).
Results
In this study, 17 women were included, which were distributed in different groups as described before. In Table 1 may be observed that the patients do not show statistical differences in age, weight, and height between the groups. AMH showed a numerical reduction when “Control” vs “Amenorrhea” were compared (1.9±0.2 vs 0.1±0.1), and a statistically significant reduction when “Control” vs “POI/OA” were compared (1.9±0.2 vs 0.6±0.1, p≤0.05) (Table 1).
Table 1. General data of patients| Group | CONTROL | POI/OA | AMENORRHEA |
| n | 6 | 8 | 3 |
| Age (years, X̅±SE) | 38±0.4 | 40±0.7 | 40±5.8 |
| Weight (kg, X̅±SE) | 53.3±4.2 | 60.4±3.6 | 59.5±3.9 |
| Height (cm, X̅±SE) | 1.54±0.008 | 1.62±0.01 | 1.61±0.03 |
| AMH (ng/mL, X̅±SE) | 1.9±0.2 | 0.6±0.1* | 0.1±0.1 |
In ultrasound results (Graph 1) it may be observed that F2 keep similar values between CONTROL, POI/OA (PRE AD-MSCs), and POI/OA (POST AD-MSCs) (7.3, 7.6, and 7.9), and AMENORRHEA (POST AD-MSCs) shows a little reduction of F2 when compared to AMENORRHEA (PRE AD-MSCs) (2 vs 1.4). With respect to F11, a statistically significant reduction may be observed in POI/OA (PRE AD-MSCs) compared with the CONTROL (9.5 vs 5.2, p≤0.05), in turn, F11 in POI/OA (POST AD-MSCs) showed a statistically significant increase when compared to POI/OA (PRE AD-MSCs) (5.2 vs 9, p≤0.05). Parallelly, AMENORRHEA (POST AD-MSCs) showed a reduction of F11 when compared with AMENORRHEA (PRE AD-MSCs) (3.3 vs 1.6). In the case of FS2, in Graph 2 may be observed a reduction in AMENORRHEA (PRE AD-MSCs) when compared with the CONTROL (2.5 vs 4.3mm). Also, in Graph 2 a statistically significant reduction of F11 in AMENORRHEA (PRE AD-MSCs) when compared to CONTROL (14.8 vs 3.7mm, p≤0.05) may be observed. By contrast, AMENORRHEA (POST AD-MSCs) keeps an increase of FS11 when compared with AMENORRHEA (PRE AD-MSCs) (3.7 vs 9.1mm).
Graph 1.Follicle to blastocyst in different groups of patients (X̅±SD).
Graph 2.Follicle size on days 2 and 11 (FS2 and FS11) in the different groups of patients (X̅±SE).
With respect to the results of the assisted reproduction lab, it can be observed that NO, MII, and OF showed values larger in CONTROL when compared to POI/OA (Pre AD-MSCs) (6.8, 4.8, and 4 vs 3.5, 2.6, and 1.3) and AMENORRHEA (Pre AD-MSCs) (6.8, 4.8 and 4 vs 0, 0, and 0). On the other hand, POI/OA (POST AD-MSCs) showed an increase of NO, MII, when compared to POI/OA (Pre AD-MSCs) (6.2, 4.2 y 2.5 vs 3.5, 2.6, and 1.3), likewise, NO, MII and OF in AMENORRHEA (POST AD-MSCs) showed a small increase compared to AMENORRHEA (PRE AD-MSCs) (1, 1 and 1 vs 0, 0 and 0). With respect to NB, POI/OA (PRE AD-MSCs) and AMENORRHEA (PRE AD-MSCs) showed a reduction when compared to CONTROL (0, 0 vs 2.5). POI/OA (POST AD-MSCs) showed an increase in NB when compared to POI/OA (PRE AD-MSCs) (3 vs 0), and AMENORRHEA (PRE and POST AD-MSCs) did not show NB (0 vs 0) (Graph 1).
In turn, the findings in patients are resumed in Table 2. It may be observed that AMENORRHEA (Pre AD-MSCs) showed less patients with follicle on day 2 (100 vs 66.6%) than the CONTROL group. In addition, AMENORRHEA (Pre and POST AD-MSCs) showed less patients with follicles on Day 11 compared to the CONTROL group (100 vs 66.6 and 66.6%). On the other hand, there were less couples that showed oocytes and MII in POI/OA (Post AD-MSCs), AMENORRHEA (Pre AD-MSCs), and AMENORRHEA (POST AD-MSCs), when compared to the CONTROL group (87.5, 0, 33.3%vs 100%).
Table 2. Development of patients by Group, from follicles on Day 2 to the outcome of pregnancy rate| CONTROL | POI/OA | AMENORRHEA | |||
| Pre AD -MSCS | Post AD-MSCs | Pre AD -MSCS | Post AD-MSCs | ||
| Follicles on Day 2 % (n) | 100 (6/6) | 100 (8/8) | 100 (8/8) | 66.6 (2/3) | 100 (3/3) |
| Follicles on Day 11 % (n) | 100 (6/6) | 100 (8/8) | 100 (8/8) | 66.6 (2/3) | 66.6 (2/3) |
| Oocytes, % (n) | 100 (6/6) | 100 (8/8) | 87.5 (7/8) | - | 33.3 (1/3) |
| MII, % (n) | 100 (6/6) | 75 (6/8) | 87.5 (7/8) | - | 33.3 (1/3) |
| Fertilized eggs, % (n) | 66.6 (4/6) | 66.6 (4/6) | 42.8 (3/7) | - | - |
| Blastocysts, % (n) | 100 (4/4) | 0 (0/8) | 100 (3/3) | - | - |
| PET, % (n) | 100 (4/4) | - | 100 (3/3) | - | - |
| PTET, % (n) | 75 (3/4) | - | 100 (3/3) | - | - |
| PAP, % (n) | 50 (3/6) | - | 37.5 (3/8) | - | - |
| MCR, % (n) | - | - | - | - | 100 (3/3) |
Oocytes (MII, MI, and germinal vesicle), PET (patients with embryo transfer), PTET (Pregnancy rate of patients with embryo transfer), PAP (pregnancy rate for all patients) y MCR (menstrual cycle recovery).
(Table 2) shows less patients with oocytes successfully fertilized in POI/OA (POST AD-MSCs), AMENORRHEA (PRE AD-MSCs), and AMENORRHEA (POST AD-MSCs) when compared to the CONTROL group, and POI/OA (PRE AD-MSCs) (42.8, 0 y 0% vs 66.6 and 66.6) (Table 2). After oocyte fertilization, 100% of the embryos turned to blastocytes in the CONTROL and POI/OA (Post AD-MSCs) groups, whereas the rest of couples POI/OA (PRE AD did not succeed to turn to blastocytes. In addition to the above, POI/OA (Post AD-MSCs) group showed a higher rate of PTET than CONTROL (75 vs 100%) group, and CONTROL group showed a higher rate of PAP than POI/OA (Post AD-MSCs). In addition, 100% of the patients in the AMENORRHEA (POST AD-MSCS) group showed temporary menstruation.
Finally, when endometrial thickness was analyzed on Day 2, it was observed that CONTROL, AMENORRHEA (PRE AD-MSCs), and AMENORRHEA (POST AD-MSCs) groups showed a reduction when compared to POI/OA (PRE AD-MSCs), and POI/OA (POST AD-MSCs) groups (2.5, 2 and 2.3 vs 3.4 and 3.7mm) (Graph 3). Later, on Day 11, endometrial thickness showed a reduction in POI/OA (PRE AD-MSCs), and POI/OA (POST AD-MSCs); AMENORRHEA (PRE AD-MSCs), and AMENORRHEA (POST AD-MSCs), when compared to the CONTROL group (8.6, 9.4, 3.5 and 6.5 vs 10.3mm). Only the AMENORRHEA (PRE AD-MSCs) group showed a statistically significant difference when compared to the CONTROL group control (10.3 vs 3.5, p≤0.05). POI/OA (POST AD-MSCs) group showed a numerical increase when compared to POI/OA (PRE AD-MSCs) group (9.4 vs 8.6mm). Similarly, the AMENORRHEA (POST AD-MSCs) group showed an increase when compared to the AMENORRHEA (PRE AD-MSCs) group (6.5 vs 3.5mm) (Graph 3).
Graph 3.Shows the endometrial thickness in the different groups of patients (X̅±SD).
Discussion
Currently, a great deal of studies have shown how MSCs help to reduce the symptoms and alterations observed in different pathologies through animal models and clinical assays. All of this, in turn, has allowed the development of better obtention techniques of MSCs from different tissues. In the case of POI, studies of transplants have shown the therapeutic potential by restoring the ovarian structure and function 59. All of the above has offered the opportunity to carry out studies like this one, which pretends to disclose our experience with the use of AD-MSCs as a coadjuvant in the treatment of women with low ovarian response.
Turning to substance, on Day 2 of menstrual cycle it was observed that the application of AD-MSCs did not increase the number of follicles in patients with infertility caused by POI or OA (7.6 vs 7.9). In addition, patients with amenorrhea, even after the application of AD-MSCs, showed a reduction in the number of follicles (2 vs 1.4). On the other hand, the application of AD-MSCs increased the number of follicles on Day 11 in patients with infertility by POI and OA (5.2 vs 9) (Graph 1). Similarly, Herraiz, 2018 60 in a group of 17 patients with low response, observed that after applying 50x106 BM-MSCs to the ovary by catheterization from Day 2 to Day 43, the number of antral follicles (3 vs 8) was increased. Volkova, 2017 61, observed an increase of the number of follicles on Day 21 in 18-week old female mice with chronic oophoritis after an intravenous infusion of BM-MSCs compared to what was observed on Day 10 (9.4 vs 15.3). Similarly, Herraiz,2018 62, reported an increase in the number of follicles from Day 7 to Day 14 (0.24 vs 0.8 follicles/mm3, in histological cuts) after the application of bone-marrow MSCs to immunodeficient mice with ovarian damage induced by chemotherapy; and Fouad, 2015 63, found a higher number of follicles after application of AD-MSCs (4 vs 13) and placental MSCs (4 vs 18) (by intravenous route) to 48 female rats with POI induced by cyclophosphamide. The effect of AD-MSCs on the increment of the number of follicles may also be reflected in the increase of follicles size on Day 11 of the menstrual cycle (POI and OA (13.5 vs 15.7mm) and amenorrhea (3.7 vs 9.1)) (Graph 2). Similarly, ABD-ALLAH, 2013 64 observed a higher number of follicles >70μm after the application of BM-MSCs (X̅:53.9 vs 16) (by intravenous route), in histological cuts from 35 female rabbits with POI induced by cyclophosphamide.
With respect to the results obtained in the laboratory of assisted reproduction, an increase of Total Oocytes (3.5 vs 6.2) was found in patients with POI and OA, which allowed to have a greater number of oocytes in “MII” (2.6 vs 4.2) after the application of AD-MSCs. In addition, patients with amenorrhea increased the possibility to show at least one mature oocyte (0 vs 1) (Graph 1). Similarly, Luján 2020 2, in a case report of a woman (39 years old) diagnosed with poor ovarian response, the application of AD-MSCs (intraovarian) increased the number of MII oocytes obtained (3 to 14). Also, Herraiz,2018 62, reported an increment of the number of MII oocytes (15 vs 30) in immunodeficient mice with ovarian damage induced by chemotherapy after the application of BM-MSCs, and Li, 2018 65 showed a higher number of follicles (11 vs 39) in a group of female mice with POI induced by cyclophosphamide, six weeks after the application of MSCs obtained from a chorionic plate. On the other hand, Herraiz, 2018 60, found that the application of 50x106 BM-MSCs by catheterization in ovary, in a group of patients diagnosed as poor responders, did not increase the amount of MII oocytes (2 vs 2). So far, it can be observed that the benefit of AD-MSCs is higher in patients with POI and OA than in patients that do have menstrual cycle anymore. This might be improved by using a method with a longer administration of MSCs (trying several different doses) as it was observed that ovaries responded very shortly.
After the intracytoplasmic spermatozoid injection (ICSI) to the oocytes collected, an increase of the number of “fertilized oocytes” was observed after the application of AD-MSCs in patients with POI, OA (50 to 59.5%) and amenorrhea (0 vs 100% corresponding to 1 patient out of 1) (Graph 1), which agrees with Zafardous, 2020 66, who found an increase of the number of “fertilized oocytes” after applying MSCs derived from menstrual blood (men-MSCs) (76 vs 92%) in 15 patients with POI. This allowed patients with POI and OA to show a higher number of blastocysts after the application of AD-MSCs, which coincides with Zafardous, 2020 66, who report an increase of 0 to 3 blastocysts in patients with POI. Patients with amenorrhea did not show any blastocysts.
In Table 2 it is clear how patients with POI and OA get benefits from the administration of AD-MSCs as an adjuvant for low ovarian response as evidenced by a higher number of patients showing MII oocytes, blastocysts, and that got pregnant (0 vs 3). The number of patients that restore temporary their menstrual cycle increases and so their possibility to obtain at least one mature oocyte, which is concordant with Edessy M, 2015 67, who report two menstruation recoveries and only one pregnancy in a group of 10 patients with POI after the application of BM-MSCs. In turn, Gupta S, 2018 68, in a case report of a 45 year old patient with infrequent menstruation for at least three years, found that the application of BM-MSCs lead to obtain three oocytes, which allowed a successful pregnancy and the birth of a baby girl.
In addition, when endometrial thickness was measured, an increase was found on Day 11 of menstrual cycle in patients with POI, OA (8.6 to 9.4mm) and with amenorrhea (3.5 to 6.5 mm) after the administration of AD-MSCs, which might be associated to the recovery of the ovarian function and an endocrine communication existing between ovaries and uterus 67, 69, 70, 71. Tersoglio, 2020 72, found similar effects of MSCs when administered directly in uterus in a study where an increase in endometrial thickness (5.2 a 9.9mm) was reported in 29 patients with thin endometrium to which endometrial MSCs (en-MSCs) were administered. On the other hand, Singh, 2020 73, found that the application of BM-MSCs in endometrium increases the thickness thereof in women with Asherman's syndrome (2.6 to 4.2) and endometrial atrophy (3.6 to 5.9).
Theoretically, the results obtained in this study might reflect disturbances related to FSH such as polymorphisms of FSH receptors (FSHr), low sensitivity to FSHr induced by damage caused by free radicals, environmental contaminants, life style, radiotherapy, autoimmune response, chemotherapy, and diet, in addition to LH variants that could turned it less reactive 74, 75, 76, 77; therefore, the application of AD-MSCs might help to reduce these disturbances by generating new blood vessels 78, downregulating the immune response 79, providing a more suitable microenvironment 80, 81, and avoiding the apoptosis of several cell types derived from possible niches of the ovary 82, 83. In addition, these niches might also be responsible of generating a microenvironment in response to possible internal and external factors that may be benefic for the quiescence or differentiation (to epithelial and follicular ovarian cells) of AD-MSCs, which favors the restauration of the ovarian function 81.
Finally, notwithstanding that there is a great deal of evidence showing the benefits of MSCs as a therapy for a large number of pathologies, in the specific case of ovarian conditions, said benefits are poorly known, in addition to the scarce knowledge of the action mechanisms associated to each one of the pathologies studied so far. Therefore, it will be of great importance to carry out more studies like this that provide data to expand the knowledge about this matter.
Conclusions
The results of this study suggest that the application of AD-MSCs directly to the ovary improve the ovarian function, stimulate endometrial growth, and induce the collection of a larger number of follicles, which makes it possible to obtain more MII oocytes which, in turn, increase the possibility to obtain blastocysts of a higher quality and therefore, a pregnancy, in patients with POI or OA.
In patients with amenorrhea, the application of AD-MSCs restores menstruation temporary, and increases the possibility to collect at least one oocyte per menstrual cycle.
Additionally, the performance of more studies with the aim to find out the exact mechanisms by which AD-MSCs restore the ovarian function is suggested. This way, we will be able to observe whether there is a tendency in favor of using AD-MSCs as a therapy or treatment for POI and OA.
References
- 1.Hare J, Fishman J. (2012) Gerstenblithet al.,Comparison of Allogeneic vs AutologousBone Marrow–Derived Mesenchymal Stem CellsDelivered by Transendocardial Injectionin Patients With Ischemic Cardiomyopathym, The POSEIDON, Randomized Trial,JAMA. 308(22), 1-11.
- 2.Abd-Allah S, Shalaby S, Pasha. (2013) H.,et al.,Mechanistic action of mesenchymal stem cell injection in the treatmentof chemically induced ovarian failure in rabbits,Cytotherapy. 15, 64-75.
- 3.Alagesan S, Griffin M. (2014) Autologous and Allogeneic Mesenchymal Stem Cells in Organ Transplantation: What do We Know About Their Safety and Efficacy?,Current Opinion in Organ Transplantation. 19(1), 65-72.
- 4.Andrzejewska A, Lukomska B, Janowski M. (2019) Concise Review: Mesenchymal Stem Cells: From Roots to Boost,Stem Cells. 27, 855-864.
- 5.Baber R, Panay N, Fenton A. (2016) . IMS Recommendations on Women's Midlife Health and Menopause Hormone Therapy,Climacteric 19(2), 109-150.
- 6.Barbieri R. (2014) Human Fertility: Methods and Protocols. Methods in Molecular Biology,The Endocrinology of the Menstrual Cycle 1154, 145-169.
- 8.Buhler K, Conforti A, G De Placido, Esteves S. (2016) A New more Detailed Stratification of low Responder the Ovarian Stiulation: From a Poor Ovarian Response to a low Prognosis Concept. Fertility and Sterility. 105(6), 1452-1453.
- 9.Buigues A, Marchante M, Herraiz S, Pellicer A. (2019) Diminished Ovarian Reserve Chemotherapy Induced Mouse Model: A Tool for the Preclinical Assessmentof New Therapies for Ovarian Damage. Reproductive Sciences. 1-11.
- 10.Bulun S, Zeitoun K, Takayama K, Sasano H. (2000) Estrogen Biosynthesis in Endometriosis: Molecular Basis and Clinical Relevance,Journal of Molecular Endocrinology. 25, 35-42.
- 12.Chae J, Gavrilova L. (2018) Premature Ovarian Insufficiency: Procreative Management and Preventive Strategies,Biomedicines. 7(2), 1-10.
- 13.Chen X, Yu L, Chen L. (2019) . Menstrual Blood-Derived Mesenchymal Stem Cells Provide New Insights Into the Treatment of Coronavirus Disease 2019 (COVID-19),Journal ofTraslationalMedicine 1-33.
- 14.Cho H, Lee S, Min K. (2020) . Advances in the Treatment and Prevention of Chemotherapy-Induced Ovarian Toxicity.International Journal of Molecular Sciences 1-20.
- 15.Chong P, Selvaratnam L, Abbas A, Kumarul T. (2012) Human Peripheral Blood Derived Mesenchymal Stem Cells Demonstrate Similar Characteristics and Chondrogenic Differentiation Potential to Bone Marrow Derived Mesenchymal Stem Cells,Journal ofOrthopaedicResearch. 30(4), 634-642.
- 16.Corona T, Halabe J, Vázquez G.. Academia Nacional de Medicina de México, Recuperado el 23 de abril de 2020,http://anmm.org.mx/actas2019/SO-08-mayo-2019.pdf .
- 17.Cox L, Liu J.Primary Ovarian Insufficiency: An Update,International. , Journal of Women’s Health 2014, 235-243.
- 18.Dragojević S, Vasiljević M, Jovanović A. (2019) . Premature Ovarian Insufficiency – Novel Hormonal Approaches in Optimizing Fertility,GynecologicalEndocrinoloy 36(2), 1-4.
- 19.Drummond A. (2006) The Role of Steroids in Follicular Growth.Reproductive Biology and Endocrinology. 4(16), 1-11.
- 20.Edessy M, Hosni H, Shady Y. (2016) Autologous Stem Cells Therapy. , The First Baby of Idiopathic Premature Ovarian Failure,Acta Medica International 3(1), 19-23.
- 21.Eftekhar M, Aflatoonian A, Mohammadian F, Eftekhar T. (2013) Adjuvant growth hormone therapy in antagonist protocol in poor responders undergoing assisted reproductive technology,Archives of Gynecology and Obstetrics. 287, 1017-1021.
- 22.Eftekhar M, Sadat E, Tabibnejad N. (2018) . Outcome of Reproductive Technology in Different Subgroups of Poor Ovarian Responder Fulfilling the POSEIDON Criterian, Middle East,Fertility Society Journal 23, 399-403.
- 23.Fan X, Zhang Y, Li X, Fu Q. (2020) Mechanisms Underlying the Protective Effects of Mesenchymal Stem Cell Based Therapy,Cellular and Molecular Life Sciences. 77, 2771-2794.
- 24.Fazeli Z, Abedindo A, Davood M, Hossein S. (2017) Mesenchymal Stem Cells (MSCs) Therapy for Recovery of Fertility: A Systematic Review,Stem Cell Reviews and Reports. 14(1), 1-12.
- 25.Flores E, Montesinos J, Mayani H. (2006) Células Troncales Mesenquimales: Historia, Biología y Aplicación Clínica,Revitade Investigación Clínica. 58(5), 498-511.
- 26.Fouad H, Sabry D, Elsetohy K, Fathy N. (2016) . Therapeutic Efficacy of Amniotic Membrane Stem Cells and Adipose Tissue Stem Cells in Rats With Chemically Induced Ovarian Failure,Journal of Advanced Research 7(2), 233-241.
- 27.Gugliandolo A, Bramanti P, Mazzon E. (2019) Mesenchymal Stem Cells: A Potential Therapeutic Approach forAmyotrophic Lateral Sclerosis? Stem Cells International. 1-16.
- 28.Guo Y, Yu Y, Hu S. (2020) . The Therapeutic Potential of Mesenchymal Stem Cells for Cardiovascular Diseases,Cell Death & Disease 11(349), 1-10.
- 29.Gupta S, Lodha P, Karthick M, Rajesh S. (2018) Role of Autologous Bone Marrow-Derived Stem Cell Therapy for Follicular Recruitment in Premature Ovarian Insufficiency: Review of Literature and a Case Report of World’s First Baby with Ovarian Autologous Stem Cell Therapy in a Perimenopausal Woman of Age,Journal of Human Reproductive Sciences. 11(2), 125-130.
- 31.Han Y, Li X, Zhang Y. (2019) Mesenchymal Stem Cells for Regenerative Medicine,Cells. 8(886), 1-32.
- 32.Herraiz S, Buigues A, Diaz C. (2018) Fertility Rescue and Ovarian Follicle Growth Promotion by Bone Marrow Stem Cell Infusion. Fertility and Sterility. 109(5), 908-918.
- 33.Herraiz S, Romeu M, Buigues A. (2018) Autologous Stem Cell Ovarian Transplantation to Increase Reproductive Potential in Patients Who are Poor Responders. Reproductive Endocrinology. 10(3), 496-505.
- 34.Hirshfield A. (1997) Overview of Ovarian Follicular Development: Considerations for the Toxicologist,Environmental and Molecular Mutagenesis. 29(1), 10-15.
- 35.Hmadcha A, Martin A, Gauthier B. (2020) Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Frontiers in Bioengineering and Biotechnology. 8(43), 1-13.
- 36.Huang W, Cao Y, Shi L. (2019) Effects of FSHR Polymorphisms on Premature Ovarian Insufficiency in Human Beings: A Meta-Analysis,Reproductive Biology and Endocrinology. 17(80), 1-6.
- 37.Iwase A, Nakamura T, Nakahara T. (2014) Assessment of ovarian reserve using anti-Müllerian hormone levels in benign gynecologic conditions and surgical interventions: a systematic narrative review. Reproductive Biology and Endocrinology. 12(125), 1-8.
- 38.Jeong H, Woo H, Park H.Mesenchymal Stem Cell Therapy for Ischemic Heart Disease: Systematic Review and Meta-analysis. , International Journal of Stem Cell 11(1), 1-12.
- 39.Kamal S, Ramaraju G, Shashikant M. (2020) . Management Strategies for POSEIDON Group 2,Frontiers in Endocrinology 11-105.
- 40.Karaoz E, Anti-apoptotic. (2012) Anti-inflammatory and Immunosuppressive Effects of Mesenchymal Stem Cells: Novel Concept for Future Therapies. Regenerative & Functional Medicine. 2-5.
- 41.Kerkis I, Santoro M, Wenceslau C, Glosman S. (2015) . Neural and Mesenchymal Stem Cells in Animal Models of Huntington’s Disease: Past Experiences and Future Challenges,Stem Cell Research & Therapy 6(232), 1-15.
- 42.Kodaman P. (2010) Early Menopause: Primary Ovarian Insufficiency and Surgical Menopause,Seminars in Reproductive Medicine. 28(5), 360-369.
- 43.Kumar M, Pathak D, Venkatesh S, la et. (2012) . Chromosomal Abnormalities & Oxidative Stress in Women with Premature Ovarian Failure (POF),Indian JournalOfMedical Research 135-92.
- 44.Kumar N, Manesh I. (2017) Premature Ovarian Insufficiency Atiology and Long-Term consequences. Openventio. 3(2), 45-58.
- 45.Laroye C, Boufenzer A, Jolly L, Bone. (2019) Marrow vs Wharton’s Jelly Mesenchymal Stem Cells. in Experimental Sepsis: A Comparative Study,Stem Cell Research & Therapy 10(172), 1-11.
- 46.Li J, Yu Q, Huang H, etal. (2018) Human Chorionic Plate-Derived Mesenchymal Stem Cells Transplantation Restores Ovarian Function. in Achemotherapy-Induced Mouse Model of Premature Ovarian Failure,Stem Cell Research & Therapy 9(81), 1-9.
- 47.Liu L, Hu C, Li R. (2017) Exosomes Derived from Mesenchymal Stem Cells Rescue Myocardial Ischaemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy Via AMPK and Akt Pathways,Cell Death and Disease. 43(1), 52-68.
- 48.Luborsky J, Meyer P, Sowers M. (2003) Premature Menopause in a Multi-Ethnic population Study of the Menopause Transition. Human Reproduction. 18, 199-206.
- 49.Lujan J, Guerrero J, Kava B. (2020) Autologous Mesenchymal Stem Cell Therapy in Patients with Unexplainable Low Ovarian Response: First Case. in Mexico,Journal of Medical & Advanced Clinical Case Reports 2(1), 1-4.
- 50.Maacha S, Sidahmed H, Jacob S. (2020) . Paracrine Mechanisms of Mesenchymal Stromal Cells in Angiogenesis,Stem Cells International 3-12.
- 51.Macklon N, Fauser B. (1998) Follicle Development During the Normal Menstrual Cycle,Maturitas. 30(2), 181-188.
- 52.Macklon N, Fauser B. (2001) Follicle-Stimulating Hormone and. Advanced Follicle Development in the Human,Archives of Medical Research 32(6), 595-600.
- 53.Mayani H. (2003) A Glance Into Somatic Stem Cell Biology: Basic Principles, New Concepts and Clinical Relevance,Archives of Medical Research. 34(1), 3-15.
- 54.Mendes D, Ribeiro P, Oliveira L. (2018) TherapyWith Mesenchymal Stem Cells in Parkinson Disease: History and Perspectives. The Neurologist. 23, 141-147.
- 55.Murra L, Péault B. (2015) Q&A: Mesenchymal Stem Cells — Where do They Come From and is it Important? BMC Biology. 13(99), 1-6.
- 56.Naji A, Favier B.Deschaseaux F.,et al.,Mesenchymal stem/stromal cell function inmodulating cell death. , Stem Cell Research & Therapy 10(56), 1-12.
- 57.Obinchemti T, Youta C. (2016) Bebeyet al.,Successful pregnancy with donor eggs in-vitro fertilization after premature ovarian insufficiency in a tertiary hospital in a low-income setting: a case report. Fertility Research and Practice. 2(12), 1-6.
- 58.Parente C, Blanco B, Christofolin D. (2011) The effect of hormones on endometriosis development. Minerva Ginecologica. 63, 375-386.
- 59.Persani L, Rossetti R, Cacciatore C. (2010) Genes Involved in Human Premature Ovarian Failure,Journal of Molecular Endocrinology. 45(5), 257-79.
- 60.Pittenger M, Discher D. (2019) Péault V.,et al.,Mesenchymal Stem Cell Perspective: Cell Biology to Clinical Progress,NPJ Regenerative Medicine. 4(22), 1-15.
- 61.Prockop D, Youn J.Mesenchymal Stem/Stromal Cells (MSCs): Role as. , Guardians of Inflammation,The American Society of Gene & Cell Therapy 20(1), 14-20.
- 62.Qayed M, Copland I, Galipeau J. (2017) Allogeneic Versus Autologous Mesenchymal Stromal Cells and Donor to Donor Variability,Mesenchymal Stromal Cells. 97-120.
- 63.Qin Y, Jiao X, Leigh J, Chen Z. (2015) Genetic of Primary Ovarian Insufficiency: New Developments and Opportunities,Human Reproduction Update. 21(6), 787-808.
- 64.Rad F, Ghorbani M, Mohammadi A, Habibi M. (2019) Mesenchymal stem cell-based therapy for autoimmune diseases: emerging roles of extracellular vesicles. Molecular Biology Reports . 46, 1533-1549.
- 65.Rawat S, Gupta S, Mohanty S. (2018) . Mesenchymal Stem Cells Modulate the Immune System in Developing Therapeutic Interventions,Immune Response Activation and Immunomodulation 1-24.
- 66.Rossetti D, S Di Angelo. (2019) Lukanović D.,et al.,Human umbilical cord-derived mesenchymal stem cells: Current trends and future perspectives. Asian Pacific Journal of Reproduction. 8(3), 93-101.
- 68.Si Z, Wang X.Sun C.,et al.,Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. , Biomedicine & Pharmacotherapy 2019, 1-6.
- 69.Singh N, Shekhar B. (2020) Mohanty S.,et al.,Autologous Bone Marrow-Derived Stem Cell Therapy for Asherman's Syndrome and Endometrial Atrophy: A 5-Year Follow-Up Study.Journal of Human Reproductive Sciences. 13(1), 31-37.
- 70.Sun Y, Sun X.Dyce P.,etal.,The role of germ cell loss during primordial follicle assembly: a review of current advances. , International Journal of Biological Sciences 13(4), 449-457.
- 71.Tersoglio A, Tersoglio S. (2020) Salatino D.,et al.,Regenerative Therapy by Endometrial Mesenchymal Stem Cells in Thin Endometrium with Repeated Implantation Failure. A Novel Strategy. JBRA Assisted Reproduction. 24(2), 118-127.
- 72.Timmreck L, Reindollar R. (2003) Contemporary issues in primary amenorrhea. Obstetrics and Gynecology Clinics of North America. 30, 287-302.
- 73.Torrealday S, Kodaman P, Pal L. (2017) Premature Ovarian Insufficiency - an update on recent advances in understanding and management [version 1; referees: 3 approved] - Thieme Connect,F1000Research. 6, 1-15.
- 74.Torres A, Torres J. (2018) . Climaterio y Menopausia,Revista de la Facultad de Medicina de la UNAM 61(2), 51-58.
- 75.W Mulder R Van, Kremer L. (2016) Recommendations for Premature Ovarian Insufficiency Surveillance for Female Survivors of Childhood, Adolescent, and Young Adult Cancer: A Report From. the International Late Effects of Childhood Cancer Guideline Harmonization Group in Collaboration With the PanCareSurFup Consortium,Journal of Clinical Oncology 34(28), 3440-3450.
- 76.Volkova N, Yukhta M, Goltsev A. (2017) Mesenchymal Stem Cells in Restoration of Fertility at Experimental Pelvic Inflammatory Disease,StemCells International. , ID 2014132, 1-9.
- 77.Wang A, Feng Y, Jia. (2019) H.,et al.,Application of Mesenchymal Stem Cell Therapy for the Treatment of Osteoarthritis of the Knee: A Concise Review,World Journal Stem Cells. 11(4), 222-235.
- 79.Warren B, Kinsey W, Mcginnis L. (2004) Ovarian Autoimmune Disease: Clinical Concepts and Animal Models,Cellular & Molecular Immunology. 11, 510-521.
- 80.Welt C. (2008) Primary Ovarian Insufficiency: A More Accurate Term for Premature,Clinical Endocrinology. , (OXFORD) 68(4), 499-508.
- 81.Xu W, Zheng J, Gao L. (2017) Neuroprotective Effects of Stem Cells. in Ischemic Stroke,Stem Cells International 1-7.
Cited by (4)
This article has been cited by 4 scholarly works according to:
Citing Articles:
Zahirrah Begam Mohamed Rasheed, F. Nordin, Wan Safwani Wan Kamarul Zaman, Yuen-Fen Tan, Nor Haslinda Abd Aziz - Biology (2023) Semantic Scholar
Biology (2023) OpenAlex
Biology (2023) Crossref
Luján Irastorza Jesús Estuardo, Di Silvio-López Mauricio, D. Carlos, H. Roberto, Ávila-Pérez Felipe de Jesús et al. - Obstetrics & Gynecology International Journal (2022) Semantic Scholar
Obstetrics & Gynecology International Journal (2022) OpenAlex
Obstetrics & Gynecology International Journal (2022) Crossref
