Essential Oils Antagonism Against Three Hygiene Significant Yeasts and Juice Spoilage by Saccharomyces Cerevisiae
Abstract
Antifungal antagonism of different fourteen plant essential oils was examined as natural agents against economic and hygienic effective three yeasts; the preservative efficacy of most potent anti-yeast essential oil in food sanitary was also tested. Study involved oils antifungal bioactivity screening against Saccharomycescerevisiae, Candida albicans,and Candida utilis. Study also included selection and invitro extraction of most bioactive oil, and evaluation of its antifungal minimal inhibitory concentrations (MICs). Control ofjuice spoilage by Saccharomycescerevisiae under the effect of in vitro oil extract different concentrations was also screened. Among the tested essential oils, apricot seed oil was the most bioactive anti-yeast agent. Two MIC values of apricot oil invitro extract, 12.5μgml-1 and 25μgml-1 were recorded. In juice samples, oil extract bioactivity increased gradually up to concentration 100μgml-1.Highest oil preservative ability was observed at oil concentration of and above 125μgml-1. Higher oil concentrations needed for juice preservation were found more than in vitro assay to give the same effect. Applying of apricot oil and some other plant essential oils could be used as an environmental safety mode in osmophilic food preservation and in Candidate diseases biocontrol.
Author Contributions
Academic Editor: Jong In Kim, Wonkwang University
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Sahar Yassin Ibrahim, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Antimicrobial activities of plant extracted essential oils (EOs) and their components play a detectable role in environment hygiene as alternative natural substances. EOs or aromatic plant essences are volatile and fragrant substances with oily consistency typically produced by plants. EOs have been long recognized for their antimicrobial, antiviral, insecticidal, antiparasitic, antidermatophytic, cytotoxic activities, antioxidant properties and as flavoring agents in foods 1, 2, 3, 4. They are widely used in medicine and in food industry as natural antimicrobial agents to control food-borne bacteria and other pathogenic microorganisms 5, 6, 7, 8, 9. In food industry, food preservation with natural antimicrobial products is being popularly used due to increase of consumer perception and concern regarding synthetic chemical additives 10. This supports applying of EOs as natural agents in food preservation.Apricot seed EO(Prunusarmeniaca) was rarely investigated as antimicrobial agent 8, 9. It showed a strong antimicrobial bioactivity and was recommended to be used as a natural food preservative and as an environmental safety mode of diseases control.
Yeasts are significant microorganisms that have positive and negative effects within food industry and microbiology. Some osmophilic yeasts are potent food spoilage agents responsible for large economic losses of some food products according to their ability to survive under special environmental conditions of low pH and water activity, and in the presence of some common chemical preservatives 11, 12, 13. Moreover, food-associated yeasts could be an underestimated source of infections and public health risks 14. As a result, yeasts have been considered as an important and attractive research area. Saccharomycescerevisiae is one of the most remarkable yeasts in food industries;as a starter for several common food products; as a spoilage causative microorganism of osmophilic foods 15, 16, 17.
Candida is a genus of yeasts which is the most not unusual purpose of fungal infections global. About 20 out of over 150 known species of Candida they have been found to cause problems for the health of humans. During the last three decades the number of fungal infections caused by Candida species has increased dramatically 18. Candidiasis is a mycosis caused by different Candida species, which can promote superficial and systemic opportunist diseases around the world 19. Candidautilis is anamorphic form of Pichiajadinii, known for its industrial applications and rarely associated with disease 18.
The increasing clinical importance of mycoses in veterinary medicine in addition to the emergence of more severe presentations prompts to the development of new diagnostic procedures and treatments 19, 20. Most antifungals currently available for the treatment of different clinical forms of this disease have limitations that hinder their use, which makes the search for safe, efficient antimycotic products or molecules necessary 19. Essential oils have long been utilized to help eradicate Candida from the body; moreover, many researches indicated that EOs have potent activity against Candida species 19, 21, 22, 23, 24, 25.
Current study was designed to assess the fungicidal antagonism of 14 plants commercial EOs against three economic and hygienic important yeast strains. Likewise, to select and test the most significant fungicidaloil in controlling juice spoilage caused by S. cerevisiae.
Materials and Methods
Study Design
Fourteen EOs were screened for their bioactivity against three yeast strains. Most significant bioactive EO was selected and re-extracted in vitro; then studied for its minimal inhibitory concentration (MIC) value against three yeasts and for its effectiveness in shelf-life extending of osmophilic fresh juice. Sugar cane juice was examined as a suspected suitable target environment for S. cerevisiae growth. Assessment of inhibition zone diameter, viable count (VC) and juice fermentation symptoms were the indicators of oil bioactivity.
Essential Oil and Plant Material
Commercial EOs from different fourteen plants were collected from "Al Captain Company" (Cairo, Egypt) and "Al-Ahlam for Seeds Oil" (Production Jeddah, Saudi Arabia). They were Thyme vulgaris, Nigella sativa, Prunus amygdoles, Olea europaea, Allium sativum,Syzgiumaromaticum, Aloe verabarbadensis, Mentha piperita, Ocimumbasilicum,Sinapis alba, Eucalyptus sp., Origanum vulgare, Armoracia rusticane, and Prunus. armeniace. Seeds of apricot, Pru. armeniaca, and fresh sugar cane juice were collected and immediately undergone bioassay.
Test Organisms
Identified fungal strains, Candida albicans, and Candida utiliswere kindly provided by Botany and Microbiology Department, Faculty of Science, Al Azhar Univ., Cairo, Egypt. Saccharomyces cerevisiae was local strain.
Microbial cultures were maintained on yeast extract malt extract agar (YEMEA) medium (diffco) at 4oC to be used as stock cultures.
Anti-Yeast Bioactivity Screening
Bioactivity of EOs was screened using agar-well diffusion test (ADT), growth inhibition zone evaluation diameters, YEMEA and Glucose peptone broth (GPB) (diffco) 26, 27, 28. For each target yeast strain, pre-cultured inoculum on GPB of 24 h age at 35oC incubation temperature was prepared and adjusted to final density of 104 CFU/ml. A set of sterile Petri dishes was prepared, each containing 25 ml sterile YEMEA seeded with 100 μL target yeast inoculum. A well of 1.0 cm diameter/Petri dish was aseptically made centrally in the solidified seeded YEMEA and subsequently 1.0 ml of EO under investigation was added in each well. Negative and positive controls were prepared, 1.0 ml of sterile saline solution (0.85% NaCl) instead of oil for negative control and 1.0 ml of standard anti-yeast nystatin reference (10 mg/ml sterile water) for positive one. The plates were kept at 4oC for 2-3 h to allow oil agar diffusion then incubated for 24 h at 35oC. Anti-yeast oil bioactivity was evaluated by measuring diameters of confirmed inhibition zones (included well diameter) in mm.
InVitro Extraction of Prunus Armeniaca Seed Oil
Evaluation of Minimal Inhibitory Concentration
MIC evaluation of invitro extracted apricot seed oil against each of the three yeast targets was assayed as follow: extracted oil stock solution was prepared; 1.0 gm extracted apricot seed oil was dissolved in 10 ml 5% dichloromethane 8. Oil stock solution was added to sterile YEMEB to get final concentrations (100, 75, 50, 25 and 12.5μgml-1). Agar-well diffusion test was applied as proceeded before using YEMEA medium seeded with one yeast strain; 1.0 ml of the tested concentration of oil extract was added in each well. Positive and negative controls were also involved. Triplicates of Petri dishes were performed for each concentration and controls. All Petri dishes were thereafter incubated at 35oC for 24 h. Diameters of differentiated inhibition zones in addition to well diameters were measured in mm. Diameter mean values were calculated and MIC value was specified. The MIC was determined as the lowest concentration of anti-yeast showing a zone of growth inhibition and expressed in μgml-1.
Osmophilic Juice Preservative Efficacy
Invitro extracted Pru. armeniaca seed oil was tested for its preservative efficiency against S. cerevisiae spoilage of osmophilic juice. Sugar cane fresh juice, Saccharumspp., was the target osmophilic juice. Juice sample was collected, examined biologically according to the Laboratory Methods in Food Microbiology 29 and sterilized by filtration. A set of nine flasks containing 100 ml sterile juice sample/flask was prepared and tested for sterility using sterile YEMEA medium. Extracted Pru. armeniaca seed oil stock was added to each flask to prepare seven concentrations in addition to the negative and positive controls. The seven tested concentrations were 200, 175, 150, 125, 100, 75 and 50μl oil stock/100ml sample. All flasks were then inoculated under aseptic conditions with 100μl yeast inoculum/100 ml juice sample and mixed well. A broth culture of S cerevisiae adjusted to final density of 104 CFU/ml was used as the target inoculum. Samples were incubated at room temperature and inspected daily for 10 days. Yeast juice spoilage was followed via monitoring of sample fermentation for alcohol odor and gassy appearance and evaluation of yeast VC expressed in CFU/ml on YEMEA medium. Data of juice fermentation following up and VC evaluation in juice samples were collected daily.
Statistical Analysis
After testing the data for normality, two way analysis of variance (ANOVA) was used to assess the significance of variations of yeast viable count (CFU/ml) under different incubation time and concentration of oil according to SPSS software (SPSS, 2006) 30.
Results
Anti-Yeast Bioactivity Screening
The representative three yeasts showed variable responses to the 14 tested oils as mentioned in Table 1. The widest yeast inhibition spectrum (23-25 mm) was confirmed with Pru.armeniaca EO, followed by M.piperita, S.aromaticum, O.basilicum, O. vulgare and O.europaea in ascending order. On contrast, Pru.amygdoles exhibited the lowest antifungal activity with 11-12 mm inhibition zones against three tested yeasts.
Table 1. Evaluation of growth inhibition zones diameters formed by bioactive commercial essential oils against three yeast strains| Yeast strain | |||||
| S.cerevisiae | C. albicans | C. utilis | |||
| Essential Oils | |||||
| Commom name | Latin name | Inhibition zone diameter (mm) | |||
| 1 | Apricot | Prunus armeniace | 24 | 25 | 25 |
| 2 | Mint | Mentha piperita | 18 | 24 | 24 |
| 3 | Cloves | Syzgium aromaticum | 20 | 21 | 21 |
| 4 | Basil | Ocimum basilicum | 18 | 20 | 20 |
| 5 | Oregano | Origanum vulgare | 19 | 18 | 18 |
| 6 | Olive | Olea europaea | 17 | 19 | 19 |
| 7 | Camphor | Eucalyptus sp. | 16 | 18 | 19 |
| 8 | Horseradish | Armoracia rusticane | 19 | 16 | 17 |
| 9 | Garlic | Allium sativum | 18 | 16 | 17 |
| 10 | Black cumin | Nigella sativa | 14 | 15 | 15 |
| 11 | Thyme | Thyme vulgaris | 18 | 13 | 13 |
| 12 | Mustard | Sinapis alba | 18 | 14 | 15 |
| 13 | Aloe | Aloe vera barbadensis | 13 | 19 | 19 |
| 14 | Almonds | Prunus amygdoles | 11 | 12 | 12 |
Evaluation of Minimal Inhibitory Concentration
On comparing zone diameters evaluation data in the dose response, a negative correlation between yeast growth and oil concentration value of Pru. armeniacaEO was observed; where the growth of the three yeasts were decreased with increasing oil concentration (Figure 1). As a result of achieved data, 12.5μgml-1 was recorded as the Pru. armeniacaoil MIC against C. utilis (Figure 1). While 25μgml-1 was confirmed as the Pru. armeniacaMIC against both S. cerevisiae and C.albicans.
Figure 1.Effect of different concentrations of in-vitro extracted Prunus armeniace oil on the growth inhibition zones diameters against three yeast strains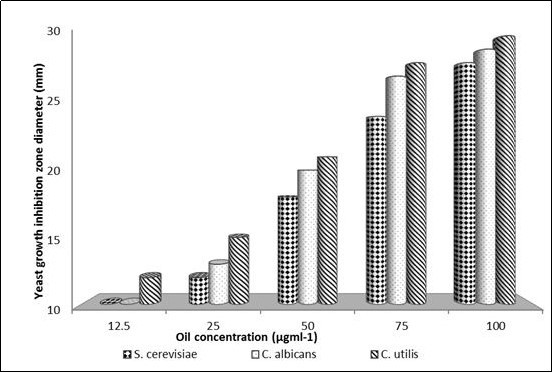
Osmophilic Juice Preservative Efficacy
Control of S. cerevisiae juice spoilage was effectively impressed byapricot oil extract at room temperature (Table 2 and Figure 2). According to estimated data, it was found that high oil concentrations were needed for effectively juice preservation against yeast fermentation even for 10 days of incubation. Also yeast VC was markedly declined at high and relatively high concentrations. At the same time, low oil extract concentrations showed low tendency in juice preservation. In negative controls of oil-free juice, yeast VC was over counting and vigorous juice spoilage symptoms were observed; extreme alcohol odor and gassy appearance appeared at the end of the second day of storage at room temperature. It was noticeable in Figure 1 and Figure 2 that the anti-yeast bioactivity of oil extract in juice samples were detected at higher oil concentrations than in vitro assay.
Figure 2.Efficiency of in-vitro extracted Prunus armeniace oil concentrations against Saccharomyces cerevisiae viable count in sugar cane juice at different incubation periods under room temperature
| Prunus armeniaceoilconcentration (µl/100ml) | Spoilage symptoms (fermentation appearance) | |||||||||||||
| Incubation period (day) | ||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 - 10 | ||||||||
| Control (0) | + a | - b | ++ a | + b | +++a | +++b | +++a | +++ b | +++a | +++b | +++a | +++b | +++a | +++b |
| 50 | + a | - b | + a | - b | ++ a | - b | ++ a | + b | ++ a | ++b | ++ a | ++b | +++a | +++b |
| 75 | -a | - b | - b | - b | + a | - b | + a | - b | + a | + b | ++ a | + b | ++ a | ++b |
| 100 | -a | - b | - b | - b | -a | - b | -a | - b | -a | - b | + a | + b | + a | +b |
| 125 - 200 | -a | - b | - b | - b | -a | - b | -a | - b | -a | - b | -a | - b | -a | - b |
+++a: strong alcohol odor ++ a: detectable alcohol odor
+a: barely alcohol odor - a: no alcohol odor
+++b: vigorous gas effervescence ++b: detectable gas effervescence
+ b: beginning of gas effervescence - b: no gas effervescence
Statistical Analysis
Statistical analysis detected high significant difference among the incubation periods, oil concentrations and the interaction between incubation period and oil concentration.
Discussion
Current study objectives were designated in accordance with several studies who examined plants derived EOs for their various biological properties. EOs were tested as natural antimicrobial active agents in food sanitary 31, 32, 33, 34, 35, 36, 37, 38 and as a natural therapeutic treatment of Candida and other dermatophyte infections 9, 18, 19, 20, 21, 22, 23, 24, 25. The antagonistic property detected for EOs from Pru.armeniace, M.piperita, S.aromaticum, O.basilicum, O. vulgare and O.europaea were also proved by Abd El Salam and Ibrahim (2014) 8 and Ibrahim and Abd El Salam (2015) 9 who studied the efficacy of different EOs against foodborne, food spoilage and pathogenic bacteria and fungi 8, 9. As well, O. vulgare recorded a remarkable antifungal activity against Candida species when investigated by Cleff et al (2010) 19. In present assay, bioactive EOs confirmed their effectiveness in controlling yeast overgrowth and inhibition of yeast formation; The EOs antagonism was clarified by Bakkali, et al (2008) 31 who worked on EOs inhibitory effect, they explained that because of the mode of EOs extraction, mostly by distillation from aromatic plants, they contain a variety of volatile molecules such as terpenes and terpenoids, phenol derived aromatic components and aliphatic component. These oil constituents are responsible for developing the antimicrobial oil activity. They also added that in eukaryotic cells, essential oils can act as prooxidants affecting inner cell membranes and organelles. Furthermore, they recorded EOs as antioxidants; and in some cases, EOs can be associated with their capacity to exert antigenotoxic effects. Burt (2004) 36 added that the hydrophobicity of EOs enables them to partition in the lipids of the cell membrane and mitochondria, rendering them permeable and leading to leakage of cell contents.
Antimicrobial activity of apricot seed oil was rarely investigated before, only two studies was published 8, 9; in agreement with these two researches, present assay concluded that apricot seed oil could be applied as natural antimicrobial and save
its juice preservation effectiveness. Also, it is expected that under refrigeration, needed EO concentrations will be lower for juice preservation than registered in this study.
food preservative agent and as an environmental safety mode of diseases.
In current results of MIC evaluation, two values were established as a result of yeast strain variability. In accordance, four MIC values of apricot oil extract were recorded by Abd El Salam and Ibrahim (2014) 8 against different microbial strains. On contrast, Ibrahim and Abd El Salam (2015) 9 registered one MIC value on testing apricot oil activity against different dermatophytes. The negative correlation deduced between Pru. armeniaca oil extract effectiveness and yeast growth was also in parallel with these two researches 8, 9; and with Bakkali et al (2008) 31 who concluded that depending on type and concentration, EOs exhibit cytotoxic effects on living cells.
In agreement with different investigations that discussed EOs effect against growth and spoilage of yeasts in drinks and foods 39, 40, 41, 42, present survey achieved noticeable bioactivities of different EOs against S. cerevisiae growth and juice spoilage. Fermented S. cerevisiae produces alcoholic odor and even gassy appearance on spoiling osmophilic drinks according to its high VC. Both of fermentation symptoms and yeast VC were used for monitoring juice spoilage progress. Screening evaluation data of oil concentration needed for controlling juice spoilage were found in accordance with many investigators results; Burt (2004) 36, who searched antibacterial properties of EOs and their and potential applications in foods, recorded that a higher concentration is needed to achieve the same effect in foods; Bassolé and Juliani (2012) 5 mentioned that in food systems, higher concentrations of EOs are needed to exert similar antibacterial effects as those obtained in in vitro assays; Abd El Salam and Ibrahim (2014) 8 tested in vitro extracted Pru. armeniaca oil against microbial spoilage of raw foods, they estimated high oil concentrations needed for raw food preservation more than needed in vitro assay. Spoilage incidents caused by yeasts are controlled by many preservative systems as were discussed by Stratford and James (2003( 42; chilled storage effect on extending the open shelf life of foods and fruit juices were examined in variuos searches; Ghalfi et al. (2007) 37 examined EOs effectiveness in pork meat during cold storage; Belletti et al. (2008) 38 applied refrigeration in addition to EO for efficacy enhancement in fruit-based salads preservation during storage. As a result, it is recommended to use refrigeration with apricot oil extract to enhance
Conclusion
P.armeniaca, M.piperita, S.aromaticum, O.basilicum, O. vulgare and O.europaea EOs are strong yeast growth inhibitors against important economic and hygienic strains, S. cerevisiae, C. albicans and C. utilis. Apricot, P.armeniaca, seed oil are the most potent whose oil in-vitro seed extract is bioactive at low concentrations. Apricot seed oil extract is also a strong preservative agent against osmophilic juice spoilage via S. cerevisiae. So, it is recommended to use apricot seed oil and some bioactive EOs as natural bio-products for osmophilic food preservation and as a safety mode of candidiates control instead of chemotherapy.
Acknowledgements
The author gratefully acknowledges members of Microbiology and Botany Department, Al Azhar University for their generous assistance in providing Candida strains included in this work.
References
- 1.F S Santos, Novales M G M. (2012) Essential oils from aromatic herbs as antimicrobial agents. , Current Opinion in Biotechnology 23, 141-136.
- 2.Abuduni A M, Hafidi H, Algabr M, Akachar J, Almahbashi H. (2017) Evaluation of essential oils for antimicrobial activity from some Moroccan aromatic plants medicinal. , J. Mater. Environ. Sci 8(12), 4240-4245.
- 3.Bajalana I, Rouzbahania R, A G Pirbaloutib, Maggid F. (2017) Antioxidant and antibacterial activities of the essential oils obtained from seven Iranian populations of Rosmarinus officinalis. , Industrial Crops & Products 107, 305-311.
- 4.Alcoba A E T, demelo D C, P M deAndrade, H J Dias, M C Pagotti. (2017) Chemical composition and in vitro antileishmanial and cytotoxic activities of the essential oils of Ocotea dispersa (Nees) Mez and Ocotea odorifera (Vell) Rohwer (Lauraceae) Natural Product Res. https://doi.org/10.1080/14786419.2017.1385007
- 5.Bassolé I H N, H R Juliani. (2012) . Essential Oils in Combination and Their Antimicrobial Properties. Mol 17(4), 4006-3989.
- 6.Oroojalian F, Kasra-Kermanshahi R, Azizi M, M R Bassami. (2010) Phytochemical composition of the essential oils from three Apiaceae species and their antibacterial effects on food-borne pathogens. Food Chem. 120(3), 765-70.
- 7.Gutierrez G, Barry-Ryan C, Bourke P. (2009) Antimicrobial activity of plant essential oils using food model media: efficacy, synergistic potential and interaction with food components. School of Food Science and Environmental Health, Dublin Institute of Technology , Ireland .
- 8.Abd El-Salam MM, S Y Ibrahim. (2014) Antimicrobial properties of 39 essential oils against thirteen foodborne microorganisms; efficacy and environmental hygiene of Prunus armeniaca in raw food preservation under cold storage. , J Environ. Occup. Sci 3(3), 169-162.
- 9.S Y Ibrahim, Abd El-Salam MM. (2015) Anti-dermatophyte efficacy and environmental safety of some essential oils commercial and in vitro extracted pure and combined against four keratinophilic pathogenic fungi. , Environ. Health Prev. Med 20, 286-279.
- 10.R A Holley, Patel D. (2005) Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. , Food Microbiol 22(4), 273-92.
- 11.Querol A. (2006) The yeast handbook. Yeasts in food and beverages. Fleet GH (eds). Verlag Berlin Heidelberg,Springer 8-7.
- 12.C L Gerez, Torino M I, M D Obregozo. (2010) Font de Valdez G. A ready-to-use antifungal starter culture improves the shelf life of packaged bread. , J. Food Prot 73(4), 762-758.
- 13.Serpaggi V, Remize F, Grand A S L, Alexandre H. (2010) Specific identification and quantification of the spoilage microorganism Brettanomyces in wine by flow cytometry: a useful tool for winemakers. , Cytom 77(6), 499-497.
- 14.G H Fleet. (2007) Yeasts in foods and beverages: impact on product quality and safety. , Curr. Opin. Biotechnol 18(2), 175-170.
- 16.Swanson K M J. (2011) . International Commission on Microbiological Specifications for Foods (ICMSF). Milk and Dairy Prod 2, 327-305.
- 17.Xi-Lin X, Guang-Li F, Hong-Wei L, Xiao-Feng L, Guang-lei Z. (2014) Isolation, identification and control of osmophilic spoilage yeasts in sweetened condensed milk. , Afr. J. Microbiol. Res 8(10), 1039-1032.
- 18.Scoppettuolo G, Donato C, E De Carolis, Vella A, Vaccaro L. (2014) Candida utilis catheter-related bloodstream infection. , Med. Mycol. Case Rep 6, 72-70.
- 19.M B Cleff, A R Meinerz, Xavier M, L F Schuch, Meireles M C A. (2010) In vitro activity of Origanum vulgare essential oil against Candida species. , Brazilian J. of Microbiol. São Paulo: 41(1).
- 20.J M Cohen, M W Ross, Busschers E. (2008) Diagnosis and management of Candida utilis infectious arthritis in a Standardbred filly. Equine vet. , Educ 20(7), 352-348.
- 21.Khan M S A, Ahmad I. (2012) Biofilm inhibition by Cymbopogon citratus and Syzygium aromaticum essential oils in the strains of Candida albicans. , J. of Ethnopharmacol 140(2), 423-416.
- 22.A K Tyagi, Malik A. (2010) Liquid and vapour-phase antifungal activities of selected essential oils against Candida albicans: Microscopic observations and chemical characterization of Cymbopogon citratus. , BMC Complement. and Alter. Med 10, 65.
- 23.Rojas C, Francisco D, deSouza C R F, W P Oliveira. (2014) Clove (Syzygium aromaticum): a precious spice. Asian Pacific J. of tropical biomedicine. 90-96.
- 24.Szweda P, Gucwa K, Kurzyk E, Romanowska E, F K Dzierżanowska. (2015) Essential Oils, Silver Nanoparticles and Propolis as alternative agents against fluconazole resistant Candida albicans, Candida glabrata and Candida krusei Clinical Isolates. , Indian J. Microbiol 55(2), 175-83.
- 25.Abe S, Sato Y, Inoue S, Ishibashi H, Maruyama N. (2003) Anti-Candida albicans activity of essential oils including Lemongrass (Cymbopogon citratus) oil and its component, citral . , Jpn J. of med. mycol 44(4), 285-91.
- 26.P R Murray, E J Baron, M A Pfaller, F C Tenover, R H Yolke. (1999) Manual of clinical microbiology. Antibacterial susceptibility tests: dilution and disk diffusion methods. 7th edition.Washington:ASMPress
- 27.S Y Ibrahim. (2002) Microbiological and Biochemical Studies on Bioactive Actinomycete Products. PhD Thesis.Egypt:BotanyDepartment,FacultyofWomenforArts,Science,Education,AinShamsUniversity
- 28.S Y Ibrahim, Abd El-Salam MM. (2016) Isolation of anti-fungal agent from a soil inhabitant Streptomyces albaduncus-M51 and its efficacy against osmophilic food spoilage by Saccharomyces cerevisiae. , J. of Environ. and Occup. Sci 5(2), 46-38.
- 29.W F Harrigan. (1998) Laboratory methods in food microbiology. 3rd edition. , New York:AcademicPress
- 31.Bakkali F, Averbeck S, Averbec D, Idaomar M. (2008) Biological effects of essential oils – A review. , Food and Chemical Toxicology 46, 475-446.
- 32.Donsì F, Annunziata M, Sessa M, Ferrari G. (2011) Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. Food Sci. , and Technol 44, 1914-1908.
- 33.Gutierrez J, Barry-Ryan C, Bourke P. (2009) Antimicrobial Activity of Plant Essential Oils Using Food Model Media: Efficacy, Synergistic Potential and Interaction with Food Components. , Food Microbiol 26(2), 142-50.
- 34.V K Bajpai, Rahman A, S C Kang. (2008) Chemical composition and inhibitory parameters of essential oil and extracts of Nandina domestica Thunb. to control food-borne pathogenic and spoilage bacteria. , Int. J. of Food Microbiol 125(2), 117-22.
- 35.Oussalah M, Caillet S, Saucier L, Lacroix M. (2006) Antimicrobial effects of selected plant essential oils on the growth of a Pseudomonas putida strain isolated from meat. Meat Sci.73: 236-44.
- 36.Burt S. (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. , Int. J. of Food Microbiol 94(3), 223-53.
- 37.Ghalfi H, Benkerroum N, Doguiet D D K, Bensaid M, Thonart P. (2007) Effectiveness of cell-adsorbed bacteriocin produced by Lactobacillus curvatus CWBI-B28 and selected essential oils to control Listeria monocytogenes in pork meat during cold storage. , Lett. in Appl. Microbiol 44(3), 268-73.
- 38.Belletti N, Lanciotti R, Patrignani F, Gardini F. (2008) Antimicrobial Efficacy of Citron Essential Oil on Spoilage and Pathogenic Microorganisms in Fruit-Based Salads. , J. of Food Sci 73(7), 331-38.
- 39.Hazan R, Levine A, Abeliovich H. (2004) Benzoic Acid, a Weak Organic Acid Food Preservative, Exerts Specific Effects on Intracellular Membrane Trafficking Pathways in Saccharomyces cerevisiae. , Appl. and Environ. Microbiol 70(8), 4457-4449.
- 40.Saranraj P, Geetha M. (2012) Microbial Spoilage of Bakery Products and Its Control by Preservatives. , Int. J. of Pharm. & Biol. Arch 3(1), 48-38.
Cited by (3)
This article has been cited by 3 scholarly works according to:
Citing Articles:
Foods (2022) Crossref
Liyuan Niu, Jingfei Liu, Xinpei Wang, Zihao Wu, Qisen Xiang et al. - Foods (2022) Semantic Scholar
Foods (2022) OpenAlex
Journal of Human Health Research (2017) OpenAlex
