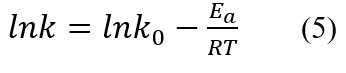Production of dried tomato powder with a high concentration of functional components and nutrients
Abstract
The use of conventional drying methods for dehydrating vegetables and fruits with high sugar, amino acid, and moisture content is not feasible. Under extreme conditions, drying reduces the amount of functional ingredients present in these vegetables and fruits. Recently, there has been an increasing demand for powdered tomatoes because of their nutritional and functional components. This study aimed to evaluate tomato drying under normal pressure and low-temperature conditions, to efficiently reduce water content without compromising on product quality. A convection dryer, most widely used in food factories, was used in this study. This equipment uses a low temperature zone not normally used for drying, and processes the raw material to increase the drying area for rapid drying. The proposed method was validated by comparing the moisture and functional component contents, and the antioxidant activity of the dried product with those of the dried product obtained via freeze-drying. The results suggest that the proposed low-temperature drying method can produce functional dried food at food processing sites faster than using freeze-drying, with a residual rate of functional ingredients exceeding 90 %. Thus, low-temperature drying can be used as a simple and cost-effective method for the production of uniform dry tomato powder.
Author Contributions
Academic Editor: Abdelmonem Awad Mustafa Hegazy, Professor and Former Chairman of Anatomy and Embryology Department, Faculty of Medicine, Zagazig University, Egypt
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2023 Masayo Nishizono, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Kumamoto is an agricultural prefecture located in the central part of Kyushu, Japan. It ranks fifth in agricultural output across the country with cauliflower, tomatoes, watermelon, eggplants, and ginger among its high-yielding crops 1. As a part of the Sixth Industrialization initiative for Japan’s agriculture, forestry and fisheries support policy, the prefectural government of Kumamoto integrates the production, processing, distribution, and sale of various agricultural resources in the prefecture 2. In this study, we focused on the processing sector, and the drying processes in particular.
Drying is a crucial step in food processing because the reduction in the water content of food prevents spoilage and degradation caused by microorganisms, enzymes, and chemical reactions. This process allows for the prolonged storage of food and its subsequent distribution to distant locations. In Japan, as early as the Edo period (1600–1868), drying has been employed to ginger and perilla for medicinal use. Currently, dried food, or kanbutsu in Japanese, has gained popularity because it offers the benefit of being ready-to-eat or ready-to-cook while retaining its nutrient content. Dried vegetables, in particular, can be used to address the low consumption of vegetables in Japan, which is currently at 20 % of the target value. Advancing from the simple technique of sun-drying, food dehydration has become increasingly sophisticated over the years. The innovations made in drying technology are usually driven by the increasing demand for processed products.
Owing to its simple operation, low cost, and high versatility 3, hot-air drying is one of the most common technologies for the dehydration of fruits and vegetables. Previous studies have reported the effect of hot-air drying on agricultural products, such as tomatoes 4 and apples 5. Browning 6 and long drying times 7, 8 are some of the drawbacks of this technique, which are mainly associated with uneven drying of the product, starting from the surface. The surface hardens before heat reaches the product core; thus, most of the water inside the product remains intact 9.
Other food drying techniques include vacuum drying, microwave drying 10, 11, and freeze-drying (FD) 12. These techniques require specialized equipment and incur high operating costs. However, the product quality obtained is better than that obtained using hot-air drying, primarily because hot-air drying is often performed at temperatures between 50 and 70 °C 13, 14, 15, 16. FD, in particular, is advantageous in maintaining product quality in terms of the color, taste, aroma, and nutritional value of the raw product 12. In this study, various tomato cultivars, produced in Kumamoto, were dehydrated using a convection dryer typically used in hot air-drying. Furthermore, FD was used as a reference to compare its performance with low-temperature drying (LTD).
In agricultural processing, the commercialization of dried processed goods has become increasingly popular because of their convenience and long shelf-life. Notably, Kumamoto records the highest production volume of tomatoes in Japan. Recently, there has been an increasing demand for powdered tomatoes because of their nutritional and functional components. In this study, we also investigated the effect of the ripening level of tomatoes on the nutritional content of the powdered product. Since large quantities are often discarded as a consequence of fruit thinning, there has been a significant demand for the effective utilization of unripe tomatoes; therefore, the nutritional content of powdered green tomatoes was also evaluated in this study.
Materials and methods
Materials
In this study, the following three types of mature fruits (red tomatoes) were considered Animo TY12, Furinkazan, and Housakukigan 1103. In addition, the following four types of immature fruits (green tomatoes) were included: Animo TY12, Furinkazan, Housakukigan 1103, and Momotaro (Takii & Co., Ltd., Kyoto, Japan). The tomatoes were cultivated in an agricultural plastic greenhouse at the Kumamoto Prefectural Agricultural Research Center (Koshi City, Kumamoto, Japan).
Table 1 shows the sugar, citric acid, and glutamic acid content of the different samples. Each tomato was crushed with a cup-holder type crusher in a closed vessel (Magic BULLET, MB-1001, Oak Lawn Marketing, China) using the blender option and subsequently subjected to drying tests and further analyses. The processed fruits were stored in a freezer at -80 °C for two months until further analysis.
Table 1. Nutritional content of each variety of tomato.| Variety & Maturity | Parameter | |||
| Sugar(Brix a) | Citric acid(mg% b) | Glutamic acid(mg% b) | ||
| Red | Animo TY12 | 4.6 ± 0.24 | 346.0 ± 0.13 | 137.9 ± 5.41 |
| Furinkazan | 4.3 ± 0.27 | 317.0 ± 6.21 | 108.7 ± 4.26 | |
| Housakukigan1103 | 4.5 ± 0.36 | 353.6 ± 0.15 | 126.6 ± 3.27 | |
| Green | Animo TY12 | 4.6 ± 0.23 | 502.9 ± 1.79 | 18.3 ± 0.26 |
| Furinkazan | 4.5 ± 0.20 | 476.6 ± 3.37 | 15.3 ± 0.41 | |
| Housakukigan1103 | 4.5 ± 0.18 | 412.4 ± 3.62 | 14.9 ± 0.22 | |
| Momotaro | 5.6 ± 0.31 | 421.7 ± 22.24 | 25.7 ± 1.46 | |
Drying method
LTD was performed using a compact-type food dehydrator SM10S-EH-DPC (Kihara Works Co., Ltd., Yamaguchi, Japan). Approximately 200 g (0.2 g cm-2) of each crushed tomato was loaded into a container made of parchment paper (25 cm × 40 cm) and dried at 40, 50, and 60 °C. The samples were weighed every hour until no weight change was observed at the appropriate drying temperature. For the residual test of the functional ingredients, each tomato sample was dried at 40 °C for 10 h, based on the high lycopene content obtained at this temperature. Subsequently, each dried tomato sample was crushed and used for analyses. For the FD method, approximately 200 g of each crushed tomato was dried using a VirTis Genesis Pilot Lyophilizer (SP Scientific, Pennsylvania, US), which was set for 70 h, and then crushed prior to analysis. Each drying test was performed in triplicates using both methods, and the mean and standard deviation values obtained were used for further estimations.
Measurement of total lycopene content
Lycopene content was measured using the method proposed by Ito et al. 17, with some modifications. The amount of powder equivalent to 3 g of fresh food was placed in a 50 mL amber-colored centrifuge tube with 35 mL of diethyl ether/methanol (70/30, v/v), stirred for 10 s, and then treated in an ultrasonic bath at temperatures below 25 °C for 10 min. Following this, centrifugation was performed at 1690 × g for 10 min, and the supernatant was transferred to a 100 mL amber-colored glass flask. The sedimented residue was mixed with 15 mL of diethyl ether/methanol (70/30, v/v), stirred for 10 s, and centrifuged at 1690 × g for 10 min. This procedure was performed four times. The total volume of the collected supernatants was adjusted to 100 mL using the same solvent. The diethyl ether/methanol (70/30, v/v) extract was passed through a 0.2 µm disposable filter, diluted two-fold, and the absorbance was measured at 505 nm in a covered quartz cuvette, using diethyl ether/methanol (70/30, v/v) as the blank. Using the known lycopene absorption coefficient (3150 %-1 cm-1 at 505 nm at a concentration of 1 % and an optical path length of 1 cm), the lycopene concentration in tomato (mg/100 g) was calculated using the following equation 18:
Measurement of total polyphenol content
The Folin–Ciocalteu method 19 was partially modified to measure the total polyphenol content in LTD or FD products. A fresh sample of approximately 1 g or a dried sample powder (1 g) was mixed with 2.0 mL of 80 % aqueous methanol solution, stirred for 10 s, and treated in an ultrasonic bath at 40 °C for 10 min. Further, each stirred solution was centrifuged at 1690 × g for 10 min, and the supernatant was collected. The sedimented residue was mixed with 2.0 mL of 80 % methanol solution, stirred for 10 s, and then centrifuged at 1690 × g for 10 min. This procedure was repeated twice. The total volume of the collected supernatants was adjusted to 10 mL, and then analyzed for polyphenol content.
The total polyphenol content was quantified by adding Folin-Ciocalteu’s reagent solution and saturated sodium carbonate, developing colors, which were measured at an absorbance of 760 nm, where the total polyphenol content was calculated as the gallic acid equivalent per 100 g of dry weight (mg GAE/100 g-dry weight), as presented in the method by Sawai et al. 20.
Measurement of 1,1-Diphenyl-2-pycrilhydrazyl radical scavenging activity
A mixture of 0.2 M 2-Molphorinoethanesulfonic acid, monohydrate buffer and 400 mM 1,1-Diphenyl-2-pycrilhydrazyl (DPPH) Free Radical ethanol solution was added to the sample to develop color, and the absorbance at 520 nm was measured and quantified. The measured value was represented by an amount equivalent to Trolox, used as the standard substance as described in the work of Oki et al. 21.
Measurement of organic acids
The organic acid concentrations in each sample were measured. Approximately 100 mg of dried sample was mixed with 2 mL of 80 % aqueous methanol solution, stirred for at least 20–30 s until the mixture was homogenous, and sonicated for 10 min in an ultrasonic bath at 40 °C. The resulting solution was centrifuged at 1690 × g for 10 min, and the supernatant was collected. This procedure was repeated twice. The collected supernatant was adjusted to 10 mL and filtered using a 0.2 µm pretreatment filter. The filtrate was analyzed using high-performance liquid chromatography. The organic acid concentration in each extract was measured using a Prominence series Organic Acid Analysis System (Shimadzu Corporation, Kyoto, Japan) with two Shim-pak SCR-102H columns connected in series (Shimadzu GLC Ltd., Tokyo, Japan, 7 µm, 8.0 mm × 300 mm).
Measurement of free amino acids
The free amino acid concentration of each extract was measured using a Nexera X2 automatic precolumn derivatized amino acid analysis system (Shimadzu, Kyoto) equipped with a Kinetex EVO C18 column (Phenomenex, Inc., Torrance, California, USA; 2.6 µm, 3.0 mm × 100 mm, pore size (A): 100). Amino acid mixed standard solution type H (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) was used to prepare the calibration curve.
Statistical analyses
Two statistical tests were used in this study to determine if the results obtained were significantly different after variations in the following three factors: drying method, ripeness level, and temperature. Initially, the t-test was performed to assess the effect of two factors (i.e.,drying method and ripeness level, treated separately) on the moisture removal efficiency and the concentrations of lycopene and polyphenols in tomatoes after drying. A t-test was performed for each tomato variety, since only two means were compared at a time 22.
Furthermore, analysis of variance (ANOVA) was performed to assess if temperature had a significant effect on the concentration of polyphenols in tomatoes after drying. The one-way ANOVA was used because only a single independent variable (i.e., temperature) was investigated. Unlike the t-test, ANOVA could be used to compare the means obtained from the three temperature levels used in this study 23.
In, both, the t-test and ANOVA, the p-value approach 22 was adopted. The confidence interval was set at 95 %, where p-values less than 0.05 were considered statistically significant 24, 25. In practical terms, statistical significance implies that the factor has a significant effect on the output. All calculations were performed in MS Excel using either the T.TEST function or the “Anova: Single Factor” module of the Data Analysis tool.
Results
Changes in the weight and composition of tomatoes based on different drying temperatures
To determine the ideal temperature at which the components were less likely to depreciate, the drying test was conducted at an agricultural processing plant at temperatures of 40, 50, and 60 oC. Furinkazan was used as the test tomato sample. Figure 1 shows a graph illustrating the weight change of the tomato sample over time for drying at different temperatures. Table 2 presents the contents of polyphenols and lycopene in the samples at each drying temperature.
Table 2. Functional component concentration in tomatoes after drying at different temperatures.| Drying temperature (°C) | Amount (mg/100 g dried tomatoes) | |
| Polyphenol | Lycopene | |
| 40 | 428.1 ± 5.3 | 115.6 ± 19.8 |
| 50 | 479.2 ± 1.7 | 105.8 ± 17.6 |
| 60 | 578.2 ± 7.5 | 92.1 ± 13.8 |
As shown in Figure 1, the drying time was lesser at a temperature of 60 °C, and the weight was almost constant after approximately 3 h. Although there was a marginal difference in weight change at 50 °C, the weight remained constant at both temperatures, after 3–4 h. In contrast, the weight change after drying at 40 °C was lesser than that after drying at 60 and 50 °C, and an approximately constant weight was achieved 6 h after process initiation.
Figure 1.Drying profile of tomatoes at different temperatures.
Differences in the amounts of functional components at each drying temperature were measured using polyphenols and lycopene as representative nutrients. The polyphenol content increased with temperature. The p-value (less than 0.05) in Table 3 confirms the significant effect of temperature on the amount of polyphenols, which is consistent with the findings of Morifusa et al. (2012) 6, who stated that residual polyphenols increase with increasing temperature during the hot-air drying of cut apples, subsequently decreasing the activity of polyphenol oxidase. Since the polyphenol content remains unchanged during non-enzymatic browning 26 was inferred that the enzyme influenced this drying test as well. Therefore, it was speculated that higher the drying temperature, lesser is the activity of polyphenol oxidase and thus, higher the residual polyphenols.
Table 3. Analysis of variance test for the effect of temperature (40, 50, and 60°C) on the concentration of polyphenols in tomatoes after drying.| Source of Variation | SSa | dfb | MSc | F d | p -value | e F crit |
| Between Groups | 34,907.23 | 2 | 17,453.62 | 401.29 | 4.09×10–7 | 5.14 |
| Within Groups | 260.96 | 6 | 43.49 | |||
| Total | 35,168.19 | 8 |
Unlike polyphenols, the lycopene content exhibited the highest residual content when the samples were dried at 40 °C and tended to decrease with increasing drying temperature. The relationship between the drying temperature of agricultural products and their antioxidant capacity is known. According to Vega-Glavez et al. 27, when agricultural products are dried in a temperature range of 40–80 °C, the DPPH radical scavenging activity is the highest when the drying temperature is the lowest (40 °C). Thus, components with antioxidant potential, such as lycopene, are best preserved when drying is performed at low temperatures, owing to their poor thermal stability. Based on these results, subsequent tests were performed at the lowest temperature of 40 °C. Here, even after drying for 10 h, a large amount of lycopene, the main functional component of tomato, remained intact.
Changes in the water content of tomatoes owing to different drying methods
Table 4 shows the amount of water loss from each tomato during each drying process. The moisture removal efficiency of each tomato (%) was calculated by subtracting the weight after drying from the weight before drying and dividing it by the fresh weight of each tomato.
The amount of water lost using LTD was 92.4–94.4 %, whereas that lost through FD was 92.1–94.3 %. The largest difference between them was 0.9 % for Furinkazan (red tomato). The LTD product was also frozen. The water content reduction using both these drying processes were not significantly different. Thus, as seen in Table 4, p>0.05 indicates that the type of drying method did not significantly influence the moisture content.
Table 4. Statistical data for the effect of the drying method on the moisture removal efficiency (%) in different varieties of tomatoes.| Ripeness | Variety | Moisture removal efficiency (%) | ||
| Low T drying | Freeze-drying | p-value | ||
| Red | Animo TY12 | 94.22±0.99 | 94.24±0.53 | 0.9807 |
| Furinkazan | 93.82±0.54 | 94.28±0.35 | 0.2967 | |
| Housakukigan 1103 | 94.26±0.55 | 94.32±0.33 | 0.8923 | |
| Green | Animo TY12 | 94.44±0.49 | 94.19±0.27 | 0.4888 |
| Furinkazan | 93.31±0.70 | 93.66±0.35 | 0.5077 | |
| Housakukigan 1103 | 92.64±0.54 | 93.54±0.12 | 0.0971 | |
| Momotaro | 92.41±0.41 | 92.10±0.34 | 0.3568 | |
Total lycopene content
Lycopene, which is a type of carotenoid, is a major functional component in tomatoes with strong antioxidant properties compared to those of other carotenoids 28. Table 5 presents statistical data for the effect of the drying method on the concentration of lycopene (mg per 100 g fresh fruit weight) remaining in different varieties of tomatoes. Relative to the FD method, the lycopene content of each tomato product following LTD at 40 °C was 82, 88, and 89 % for Animo TY12, Furinkazan, and Hosakukigan 1103, respectively. In all varieties, 11 to 18% reduction was observed compared to the FD sample, but it was considered to be a good amount as a functional processing material.
Table 5. The effect of the drying method on the concentration of residual lycopene (mg/100 g fresh weight) in different varieties of tomatoes.| Variety | Lycopene (mg/100 g fresh fruit weight) | ||
| Raw fruit | LTDa | Freeze-drying | |
| Animo TY12 | 6.69 ± 0.885 | 15.17 ± 0.301 | 18.60 ± 2.013 |
| Furinkazan | 10.03 ± 1.711 | 15.68 ± 0.675 | 17.89 ± 1.447 |
| Housakukigan 1103 | 9.00 ± 0.645 | 15.67 ± 0.621 | 17.57 ± 1.212 |
Several studies have reported on the lycopene content after drying. In some studies, it was found that lycopene content after FD was significantly reduced, whereas other studies reported a significant increase in the lycopene content after oven-drying 29, 30, 31. One of the reasons for this decrease in lycopene is its oxidative degradation 32. The increase in lycopene can also be caused by the release of phytochemicals from the tomato matrix during heat treatment 33. However, lycopene is also prone to deterioration during heat treatment 34, 35. Thus, it is assumed that the effect of drying on the lycopene content depends on the environment, process conditions (temperature and time), and other factors, such as maturation stage, hardness, and tomato genotype.
In this study, there was no significant difference in the lycopene content using the LTD and FD methods. Furthermore, the lycopene content of the dried powder obtained in this study did not differ significantly from that of fresh tomato fruits.
Total polyphenol content
Unique polyphenols, with strong antiallergic activity, are known to be present on the tomato skin 36. In this study, we aimed to measure the total polyphenol content as a functional ingredient, as presented in Table 6 For mature red tomatoes, the total polyphenol content was similar among all the varieties, and the polyphenol content of FD and LTD showed the same amount in all three cultivars. Table 7 shows the statistical analysis data for the effect of ripeness on the concentration of polyphenols remaining after each of the drying methods. The p-values were lower than 0.05, demonstrating that ripeness has a more significant effect on polyphenol content than that by the variety or drying method. It has been determined that LTD retains a polyphenol content similar to that of FD 33 for green tomatoes.
Table 6. The effect of the drying method on the concentration of polyphenols (mg GAE/100 g fresh weight) remaining after drying different varieties of tomatoes| Ripeness | Variety | Polyphenol (mg GAE/100 g fresh fruit weight) | ||
| Raw fruit | Low T drying | Freeze-drying | ||
| Red | Animo TY12 | 24.52 ± 0.249 | 24.12 ± 0.541 | 24.18 ± 0.887 |
| Furinkazan | 24.33 ± 0.406 | 25.00 ± 0.829 | 24.93 ± 0.887 | |
| Housakukigan 1103 | 25.97 ± 0.190 | 24.18 ± 0.336 | 24.33 ± 0.465 | |
| Green | Animo TY12 | 17.34 ± 0.129 | 15.64 ± 0.558 | 19.19 ± 0.970 |
| Furinkazan | 19.36 ± 0.210 | 18.68 ± 0.313 | 20.06 ± 0.837 | |
| Housakukigan 1103 | 17.75 ± 0.407 | 19.40 ± 0.396 | 16.86 ± 0.318 | |
| Momotaro | 34.05 ± 1.578 | 33.70 ±0.774 | 33.70 ± 1.040 | |
| Variety | p-value | |
| Low T drying | Freeze-drying | |
| Animo TY12 | 6.10E×10–5 | 2.73×10–3 |
| Furinkazan | 1.60×10–3 | 4.67×10–4 |
| Housakukigan 1103 | 4.06×10–6 | 8.59×10–5 |
| Momotaro | --- | --- |
Wojdylo et al. 37 reported the effects of vacuum microwave drying on the phenolic and antioxidant capacity of sour cherries, and its comparison to convection drying, and vacuum freeze-drying. The results showed that the same phenolic compound content was obtained with either vacuum microwave drying or vacuum freeze-drying. Convection drying (drying temperature 50-70 °C) had the lowest retention rate. Therefore, by setting the drying temperature of the convection drying to 40 oC, it can be inferred that the irreversible oxidation process and the thermal degradation of the phenolic compounds will not occur.
Hot air drying and convection drying have been reported as drying methods for crops other than omatoes 38, 39, 40, 41. However, these results reported on their combination with other drying methods, in which case, their performance and quality were reported to be inferior. In addition, most of the test studies that used "hot air drying" used drying temperatures of 50 °C or higher, whereas there were no studies with drying tests at 40 °C. In other words, to the best of our knowledge, this paper is the first to confirm that drying is possible at a temperature of 40 ℃, and drying times can be shortened by increasing the drying area in order to maintain product quality.
DPPH radical scavenging activity
Various anti-acid components in foods exhibit inhibitory effects on the generation and action of active oxygen and free radicals 42, which can lead to cancer and myocardial infarction. Moreover, their antioxidative components are known to prevent various diseases 43. Fruits contain a large quantity of ascorbic acid, carotene vitamins, and polyphenols. Tomatoes, in particular, contain anthocyanins, ascorbic acid, and phenolic compounds, and thus have high antioxidant activity 44. Thus, the DPPH radical scavenging activity was measured to quantify the antioxidant properties of tomatoes. As a result, the DPPH radical scavenging activity in mature red fruits was found to be higher than that in immature green fruits, as shown in Table 8.
Table 8. The effect of the drying method on the concentration of DPPH (equivalent mmol Trolox/100 g fresh sample) remaining after drying different varieties of tomatoes| Ripeness | Variety | DPPH (equivalent µmol Trolox/100 g fresh weight) | ||
| Raw fruit | Low T drying | Freeze-drying | ||
| Red | Animo TY12 | 63.93 ± 1.267 | 79.76 ± 0.769 | 86.00 ± 2.472 |
| Furinkazan | 65.83 ± 1.232 | 86.27 ± 0.374 | 89.76 ± 2.990 | |
| Housakukigan 1103 | 84.94 ± 0.603 | 86.61 ± 0.472 | 92.64 ± 1.444 | |
| Green | Animo TY12 | 24.54 ± 0.765 | 41.30 ± 1.116 | 43.66 ± 2.252 |
| Furinkazan | 18.68 ± 0.0781 | 42.96 ± 0.164 | 42.09 ± 2.715 | |
| Housakukigan 1103 | 8.161 ± 0.533 | 33.60 ± 1.735 | 37.08 ± 1.425 | |
| Momotaro | 37.37 ± 4.662 | 63.86 ± 2.293 | 74.81 ± 1.607 | |
The DPPH activity of the LTD tomato was approximately 4-7% lower than that of the FD dried mature tomatoes, and 0-15% lower than that of untreated immature tomatoes. For instance, DPPH concentration was shown to be affected by the type of drying method when using red varieties of tomatoes, except for Momotaro, the concentration of DPPH in the green varieties of tomatoes was not affected by the type of drying method. Therefore, it is clear that ripeness affects the amount of DPPH remaining after LTD and FD. Consequently, we analyzed the effect of ripeness on the amount of DPPH remaining after each drying method in the red and green varieties. The obtained p-values (<0.05) in all cases, presented in Table 9, statistically confirmed that ripeness affects the DPPH concentration after LTD and FD. Antioxidant activity correlates closely with total polyphenols 45. Although the remaining DPPH radical scavenging activity was slightly inferior to that of the polyphenols in the LTD sample, its level was satisfactory as a functional processing material.
Table 9. Statistical data for the effect of ripeness on the amount of DPPH remaining after low-temperature drying or freeze-drying different varieties of tomatoes| Variety | p-value | |
| Low T drying | Freeze-drying | |
| Animo TY12 | 1.36×10–4 | 4.88×10–5 |
| Furinkazan | 1.05×10–3 | 7.61×10–4 |
| Housakukigan 1103 | 5.87×10–3 | 4.91×10–3 |
| Momotaro | --- | --- |
Organic acids
Organic and amino acids influence the taste of tomatoes, and the organic acid content is a crucial sourness factor. Therefore, the organic acid content in each sample of the two drying methods was compared. Since tomato contains more than 10 types of organic acids 46, particularly citric and malic acids, we quantified the concentrations of these two organic acids for all dried samples. Figure 2 shows the organic acid content of each. Comparing mature tomatoes (red) and unripe tomatoes (green), the total organic acid content in immature tomatoes was about 5 times higher in malic acid and 1.3 times higher in citric acid than in mature tomatoes. The organic acid content varied among cultivars, and the organic acid content of the LTD sample and the FD sample fluctuated up and down for each cultivar, and no regularity was observed. We thought that there was no big difference in the reduction of organic acid content between LTD and FD.
Figure 2.Effect of drying methods – low-temperature drying and freeze-drying – on the organic acid content of different red (mature) and green (immature) varieties of tomatoes, inconsistent trends for organic acid content were observed among the tomato varieties.
Free amino acids
Glutamic acid is a typical amino acid present in tomatoes. The umami (savor) components contain glutamic acid, which is unevenly distributed in the fruit part, and the pulp contains approximately four times as much glutamic acid as the flesh part 47. As tomatoes mature, their sweetness and umami taste increase, since the monosaccharides (such as glucose and fructose) and glutamic acid content in tomatoes increase while its citric acid content decreases. The large amount of free glutamic acid in tomato fruits enhances rather than destroying the taste of other ingredients, even when it is boiled (concentrated) with seafood or meat containing nucleic acids and amino acids 48. Moreover, the original taste of tomatoes is generally attributed to the presence of glutamic and aspartic acids 47. Tomatoes are also known to contain another amino acid, γ-aminobutyric acid (GABA), at a high concentration which helps to suppress elevated blood pressure 49. In this study, we focused on the following four types of free amino acids: GABA, glutamine, glutamic acid, and aspartic acid. In addition, we investigated the residual quantities of these free amino acids after drying. As shown in Figure 3, the aspartic and glutamic acid contents in the red mature fruits were 3–8 times higher than those in the immature green fruit. Conversely, the contents of glutamine and GABA in mature fruits tended to be lower than those in immature green fruits 50. During plant maturation, glutamine can be converted to glutamic acid 51. Furthermore, similar to previous studies, these results suggest that glutamine and GABA are involved in the increase in glutamic acid during the maturation process of tomatoes 34, 35, 36, 52, 53, 54.
Subsequently, the relationship between the amino acid content and maturity of tomatoes was studied. In mature fruits, drying did not result in a decrease in any amino acid. Additionally, in some immature green fruit varieties, amino acid content decreased to less than 50 % of those in fresh fruits. From this result, it is speculated that the amino acids present during the maturation process have unstable chemical structures, which can be degraded using any treatment, such as drying. The magnitude of change in amino acid concentrations during LTD was compared to that of FD, and it was found to be similar to that found in fresh fruits. Therefore, the results suggest that LTD does not reduce the amino acid components, thus validating the efficiency of this drying method and its ability to result in good-quality products.
Figure 3.Effect of drying methods – low-temperature drying and freeze-drying – on the amino acid content of different red (mature) and green (immature) varieties of tomatoes. GABA, γ-aminobutyric acid.
Kinetics for moisture removal rates
To investigate the advantages of LTD using physical methods, the rate of moisture removal in each drying process was calculated by dividing the water removal rate by the drying time and compared, as shown in Table 10. The rate of moisture removal by low-temperature drying was 9.241–9.444 %/h, and that of FD was 1.316–1.347 %/h. Thus, the rate of moisture removal during LTD was approximately seven times faster than that during FD. This difference is based on the operating time of each drying method. As reported in Table 10, statistical analysis revealed that there is a significant difference between LTD and FD with respect to moisture removal (p<0.05).
Table 10. Statistical analysis on the effect of the type of drying—low-temperature drying and freeze-drying—on the velocity of moisture removal in tomatoes at different ripeness levels and varieties. The velocity of moisture removal is obtained by dividing the moisture removal rate by the drying time.| Ripeness | Variety | Rate of moisture removal (%/hr) | ||
| Low T drying | Freeze-drying | p-value | ||
| Red | Animo TY12 | 9.422 ± 0.081 | 1.346 ± 0.006 | 4.532 × 10–5 |
| Furinkazan | 9.382 ± 0.044 | 1.347 ± 0.004 | 1.275 × 10–5 | |
| Housakukigan 1103 | 9.426 ± 0.045 | 1.347 ± 0.004 | 1.368 × 10–5 | |
| Green | Animo TY12 | 9.444 ± 0.04 | 1.346 ± 0.003 | 1.083 × 10–5 |
| Furinkazan | 9.331 ± 0.058 | 1.338 ± 0.004 | 2.369 × 10–5 | |
| Housakukigan 1103 | 9.264 ± 0.044 | 1.336 ± 0.001 | 1.541 × 10–5 | |
| Momotaro | 9.241 ± 0.033 | 1.316 ± 0.004 | 6.674 × 10–5 | |
A kinetic analysis for tomato drying using LTD was performed. So far, there have been various reaction kinetic models that have been proposed. In this study, the pseudo-first-order kinetic model (Eq. 2) which is the most used, and the Page model (Eqs. 3 and 4) were employed to calculate the drying kinetic parameters for tomato using the low-temperature drying method 55, 56, 57. Figure 4 shows the kinetic plots for the two methods.
where W0 is the weight of raw material g, W is the weight of the material obtained after drying g, kF is the drying constant for the first-order reaction model [h-1], t is the drying time h, MR is moisture content -, kpand n are functional parameters for the Page model, M(t) is the weight of material after drying time tg, M0 is the initial weight of raw material g, and Me is the equilibrium moisture content that can be determined at the final stage of drying g.
Figure 4.Kinetic plots of changes in (a) the first-order model and (b) the Page model. Symbols: ● 40oC, ▲ 50oC, and ◆ 60oC.
The -ln(W/W0) value should be proportional to the drying time when following the pseudo-first-order kinetic model, but it tended to be low in the early stages of drying as shown in Table 11. In particular, at 40 ℃, the correlation coefficient R2 was slightly low, at 0.94. On the other hand, in the Page model, a good correlation was confirmed between the horizontal and vertical axes at any temperature.
Table 11. Kinetic parameters for the low-temperature drying of tomato.| Model | T | K | n | R 2 | Ea | R 2 |
| °C | h-1 | kJ mol-1 | ||||
| First-order | 40 | 0.0063 | _ | 0.9472 | -35.50 | 0.9632 |
| 50 | 0.0110 | _ | 0.9934 | |||
| 60 | 0.0152 | _ | 0.9686 | |||
| Page model | 40 | 0.2859 | 1.6453 | 0.9796 | -36.39 | 0.9618 |
| 50 | 0.5084 | 1.9386 | 0.9847 | |||
| 60 | 0.6596 | 0.4161 | 1.0000 |
Next, we investigated whether the relationship between the drying constants calculated at each temperature and the drying temperature could be expressed by the Arrhenius-type rate equation (Eq. 5) or not.
where lnk0 is the preexponential factor -, Ea is the apparent activation energy [J mol-1], R is the gas constant 8.314 [J K-1 mol-1], and T is the temperature (K). Figure 5 shows the Arrhenius plot. Both drying models could be approximated by a downward sloping straight line with respect to the reciprocal of temperature. This indicates that the drying rate of tomato by the LTD method followed the Arrhenius-type drying rate law. The apparent activation energy, Ea, calculated from the slope of the straight line was about 36 kJ mol-1 for both models.
Figure 5.Arrhenius plot for the drying constants. Symbols: ● The first-order model, ▲ The Page model.
Conclusion
In summary, the preservation characteristics of polyphenols, DPPH antioxidant activity, organic acids, amino acids, and taste components obtained using the LTD process were similar to those obtained using FD. Moreover, LTD is the preferred drying method for developing functional dried products at food processing sites in a shorter time and at a lower cost than those with FD.
Using conventional drying methods, it is difficult to obtain dried powder products of vegetables and fruits with high-concentrations of sugars, amino acids, and water, such as tomatoes. Additionally, these drying methods reduce the concentration of functional components of the raw materials. Consequently, we carried out drying in a temperature range that does not reduce the concentration of functional components (LTD), and compared the results to those of FD, which preserves the raw characteristics and causes minimum changes in ingredients. We further examined the effectiveness of both methods, and arrived at the following conclusions:
After drying, the moisture content derived from both the methods was 5–8 %. In comparison, LTD was conducted for 10 h, whereas FD lasted for 70 h, which was at least seven times higher than the time spent on FD.
The total polyphenol content obtained after LTD was 84–104 % (including both red and green varieties) of that for FD; the difference in the residual content using the two methods was not statistically significant.
The DPPH radical scavenging activity, an indicator of antioxidative activity, was approximately 4–8 % for red tomatoes, 2–17 % for green tomatoes, and the activity was lower using LTD than that with FD.
he residual ratio of organic acids and free amino acids in LTD was ≥ 90 %, which was similar to that obtained with FD.
The rate of moisture removal during LTD was approximately seven times faster than that during FD.
The kinetics analysis with the pseudo-first-order reaction rate model and Page model done in this study has not been reported in other works for this type of drying and this type of fruit. Furthermore, the drying behavior at 40℃ followed both of the models, especially the Page model.
In conclusion, LTD can produce a uniform dry powder with a small decrease in the concentration of functional components even in a small food processing factory where it is difficult to introduce FD. Thus, it is possible to easily produce dry powder with a high concentration of functional ingredients equivalent to FD at a comparatively lower cost. In addition, it is also possible to handle various raw materials with high sugar or moisture content. We believe that this study on LTD produces evidence for a new drying method that can easily produce high-quality powder in a short time.
Abbreviations
LTD, Low-Temperature Drying; FD, Freeze Drying; SEM, Scanning Electron Microscope; WHC, water holding capacity; WSI, water solubility index; DPPH, 1,1-diphenyl-2-picrylhydrazyl.
Disclosure statement
The authors report that there are no competing interests to declare.
Acknowledgments
The authors would like to thank Editage (www.editage.com) for English language editing.
Funding sources
The authors did not receive support from any organization for the submitted work.
Ethics statement
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable
Authors’ contribution statements
Ms. Masayo Nishizono designed the experimental and analytical systems for this study and also discussed with the authors so that it could be summarized for the manuscript.
Ms. Cinthya Soreli Castro Issasi edited the draft of Ms. Nishizono and Dr. Mizukami had prepared on the basis of the experimental and analytical findings.
Dr. Jonas Karl Christopher N. Agutaya worked on the statistical analysis of the obtained results and the edition of this manuscript.
Prof. Mitsuru Sasaki supervised this study and discussed with the co-authors so that the results could be effectively summarized for the manuscript.
Dr. Hiroyuki Mizukami investigated related research on freeze-drying and high-temperature drying of natural plants, vegetables, and fruits, Furthermore, discussed the findings with the co-authors so that the findings could be summarized for the manuscript.
Research Highlights
A low-temperature drying method produces high-quality tomato powder.
Tomato powder made via LTD has minimal functional and nutritional component loss.
LTD uses a convection dryer, resulting in a shorter operating time than FD.
References
- 1.Prefecture Kumamoto. (2018) Agriculture, Forestry and Fisheries Policy Division. , Japan, 31.https://www.pref.kumamoto.jp/uploaded/attachment/4502
- 3.Ema M. (2006) On transition of dehydrated foods (vegetable, mushroom and fruit) by patent gazettes. , Journal for the Integrated Study of Dietary Habits 17(3), 239-246.
- 4.Doymaz I. (2007) Air-drying characteristics of tomatoes. , Journal of Food Engineering 78(4), 1291-1297.
- 5.Sjöholm I, Gekas V. (1995) Apple shrinkage upon drying. , Journal of Food Engineering 25(1), 123-130.
- 6.Morifusa S, Orikasa T, Muramatsu Y, Tagawa A. (2012) Effect of solution spraying during hot air drying on surface hardening and browning of freshly cut apple pulp. , Nippon Shokuhin Kagaku Kogaku Kaishi 59(11), 583-590.
- 7.Maskan M. (2001) Drying, shrinkage and rehydration characteristics of kiwifruits during hot air and microwave drying. , Journal of Food Engineering 48(2), 177-182.
- 8.Orikasa T, Wu L, Shiina T, Tagawa A. (2008) Drying characteristics of kiwifruit during hot air drying. , Journal of Food Engineering 85(2), 303-308.
- 9.Orikasa T, Tagawa A, Soma S, Iimoto M, Ogawa Y. (2005) Hot Air Drying Characteristics of Fruits and Vegetables and Surface Hardening of Samples during Drying. , Journal of the Japanese Society of Agricultural Machinery 67(6), 62-70.
- 10.Orikasa T, Shibata T, Nei D, Roy P, Nakamura N et al. (2008) Microwave Drying Characteristics of Sliced Radish. , Nippon Shokuhin Kagaku Kogaku Kaishi 55(7), 350-354.
- 11.Heredia A, Barrera C, Andrés A. (2007) Drying of cherry tomato by a combination of different dehydration techniques. Comparison of kinetics and other related properties. , Journal of Food Engineering 80(1), 111-118.
- 12.Yamaguchi A, Nishi R, Hirose J, Urabe K, Nadamoto T. (2012) . Changes in Food Items Because of Processing with Different Drying Methods , Food Preservation Science 38(3), 169-176.
- 13.Ando Y, Orikasa T, Shiina T, Sotome I, Isobe S et al. (2010) Application of microwave to drying and blanching of tomatoes. , Nippon Shokuhin Kagaku Kogaku Kaishi 57(5), 191-197.
- 14.Tamaki Y, Orikasa T, Muramatsu Y, Tagawa A. (2012) Effects of Vegetable Porosity Following Microwave Drying on Absorption of Dried Vegetables. , Nippon Shokuhin Kagaku Kogaku Kaishi 59(8), 401-408.
- 15.Yoshida H, Orikasa T, Koide S, Muramatsu Y, Tagawa A. (2013) Application of Brief Hot Water Soaking as a Pre-treatment in Kiwifruit Drying. , Nippon Shokuhin Kagaku Kogaku Kaishi 61, 151-159.
- 16.Rahman M, Joardder M U H, Karim A. (2018) Non-destructive investigation of cellular level moisture distribution and morphological changes during drying of a plant-based food material. , Biosystems Engineering 169, 126-138.
- 17.Ito H, Horie H. (2009) Proper solvent extraction for lycopene extraction in tomatoes and application to a rabid determination. , Bulletin of the National Institute of Vegetable and Tea Science 8, 165-173.
- 18.Nagata M, Yamashita I. (1992) Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. , Nippon Shokuhin Kogyo Gakkaishi 39(10), 925-928.
- 19.V L Singleton, J A Rossi. (1965) Colorimetry of Total Phenolics With Phosphomolybdic-Phosphotungstic Acid Reagents. , American Journal of Enology and Viticulture 16(3), 144-158.
- 20.Sawai Y, Oki T, Nishiba Y, Okuno S, Suda I et al. (2014) 1,1-Diphenyl-2-picrylhydrazyl Radical Scavenging Components of Leaf Lettuce. , Bulletin of the NARO Kyushu Okinawa Agricultural Research Center 61, 23-34.
- 21.Oki T. (2008) DPPH Radical Scavenging Activity Evaluation Method. Food Funct Eval Man Collect II (Food Funct Eval Support Cent Tech Spread Mater Rev Com Japanese Soc Food Sci Technol. 71-78.
- 22.D C Montgomery. (2013) Experiments with a Single Factor: In the Analysis of Variance. Design and analysis of experiments. 8th ed , Hoboken, NJ, USA: 65-138.
- 23.D C Montgomery. (2013) Simple Comparative Experiments. Design and analysis of experiments. (8th ed. , Hoboken, NJ, USA: 25-64.
- 24.Thai C C D, Bakir H, Doherty W O S. (2012) Insights to the clarification of sugar cane juice expressed from sugar cane stalk and trash. , Journal of Agricultural and Food Chemistry 60(11), 2916-2923.
- 25.T D Oluwajuyitan, S A Malomo, A, A O Idowu, T N Fagbemi. (2020) . Influence of Extractive Solvents on the Chemical Composition and Antioxidative Properties of Blends from Carica papaya Leaves and Alkalized Cocoa Powder , ACS Food Science & Technology 1(2), 146-151.
- 26.Zhu D, Ji B, H L Eum, Zude M. (2009) Evaluation of the non-enzymatic browning in thermally processed apple juice by front-face fluorescence spectroscopy. , Food Chemistry 113(1), 272-279.
- 27.Vega-Gálvez A, Ah-Hen K, Chacana M, Vergara J, Martínez-Monzó J et al. (2012) Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, colour, texture and microstructure of apple (var. Granny Smith) slices. , Food Chemistry 132(1), 51-59.
- 28.Ouchi A, Aizawa K, Iwasaki Y, Inakuma T, Terao J et al. (2010) Kinetic Study of the Quenching Reaction of Singlet Oxygen by Carotenoids and Food Extracts in Solution. Development of a Singlet Oxygen Absorption Capacity (SOAC) Assay Method. , Journal of Agricultural and Food Chemistry 58(18), 9967-9978.
- 29.Chang H, Lin H Y, Chang C Y, Liu Y C. (2006) Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. , Journal of Food Engineering 77(3), 478-485.
- 30.S E Bilek, Değirmenci A, Tekin İ, F M Yılmaz. (2019) Combined effect of vacuum and different freezing methods on the quality parameters of cherry tomato (Lycopersicon esculentum var. Cerasiforme). , Journal of Food Measurement and Characterization 13(3), 2218-2229.
- 31.Tan S, Ke Z, D Miao Chai, Luo Y, Li K et al.Lycopene, polyphenols and antioxidant activities of three characteristic tomato cultivars subjected to two drying methods. , Food Chemistry 338, 128062-10.
- 32.Sharma K, Maguer L. (1996) Kinetics of lycopene degradation in tomato pulp solids under different processing and storage conditions. , Food Research International 29, 309-315.
- 33.Dewanto V, Wu X, Adom K, Liu R H. (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. , Journal of Agricultural and Food Chemistry 50(10), 3010-3014.
- 34.Graziani G, Pernice R, Lanzuise S, Vitaglione P, Anese M et al. (2003) Effect of peeling and heating on carotenoid content and antioxidant activity of tomato and tomato-virgin olive oil systems. European Food Research and Technology 216(2), 116-121.
- 35.P M Choksi, V Y Joshi. (2007) A Review on Lycopene—Extraction, Purification, Stability and Applications. , International Journal of Food Properties 10(2), 289-298.
- 36.Yamamoto T, Yoshimura M, Yamaguchi F, Kouchi T, Tsuji R et al. (2004) Anti-allergic activity of naringenin chalcone from a tomato skin extractBioscience, biotechnology, and biochemistry. 68(8), 1706-1711.
- 37.Wojdylo A, Figiel A, Lech K, Nowicka P, Oszmianski J. (2014) Effect of Convective and Vacuum–Microwave Drying on the Bioactive Compounds, Color, and Antioxidant Capacity of Sour Cherries. , Food and Bioprocess Technology 7, 829-841.
- 38.Saxena A, Maity T, P S Raju, A S Bawa. (2012) . Degradation Kinetics of Colour and Total Carotenoids in Jackfruit (Artocarpus heterophyllus) Bulb Slices During Hot Air Drying. Food and Bioprocess Technology 5, 672-679.
- 39.Uribe E, Vega-Galvez A, K Di Scala, Oyanadel R, T J Saavedra et al. (2011) Characteristics of Convective Drying of Pepino Fruit (Solanum muricatum Ait.): Application of Weibull Distribution. Food and Bioprocess Technology 4, 1349-1356.
- 40.Vidinamo F, Fawzia S, M A Karim.. Investigation of the Effect of Drying Conditions on Phytochemical Content and Antioxidant Activity in Pineapple (Ananas comosus). (2022). Food and Bioprocess Technology 15(2), 10-1007.
- 41.Kong A-H, C E Zambra, J E Aguëro, Vega-Galvez A, R L Mondaca. (2012) . Moisture Diffusivity Coefficient and Convective Drying Modeling of Murta (Ugni molinae Turcz): Influence of Temperature and Vacuum on Drying Kinetics.Food and Bioprocess Technology 6(4), 919-930.
- 43.B N Ames, M K Shigenaga, T M Hagen. (1993) Oxidants, antioxidants, and the degenerative diseases of aging. Proceedings of the National Academy of Sciences of the United States of America 90(17), 7915-7922.
- 44.H M Chandra, B M Shanmugaraj, Srinivasan B, Ramalingam S. (2012) Influence of genotypic variations on antioxidant properties in different fractions of tomato. , Journal of Food Science 77(11), 10-1111.
- 45.Sun Y, Shen Y, Liu D, Ye X. (2015) Effects of drying methods on phytochemical compounds and antioxidant activity of physiologically dropped un-matured citrus fruits. LWT-Food Science and Technology 60(2), 1269-1275.
- 46.G E Hobson, J A Davies. (1971) The Tomato. In:. , London:, A. Hulme, Ed., The Biochemistry of Fruits and Their Products (Vol 2, 437-82.
- 47.Oruna-Concha M-J, Methven L, Blumenthal H, Young C, D S Mottram. (2007) . Differences in Glutamic Acid and 5’-Ribonucleotide Contents between Flesh and Pulp of Tomatoes and the Relationship with Umami Taste , Journal of Agricultural and Food Chemistry 55(14), 5776-5780.
- 49.Saito T, Matsukura C, Sugiyama M, Watahiki A, Ohshima I et al. (2008) Screening for g-aminobutyric acid (GABA)-rich Tomato Varieties. , Journal of the Japanese Society for Horticultural Science 77(3), 242-250.
- 50.Nagata M, Saijo R. (1992) Changes in free amino acid contents of tomato fruits during ripening, especially changes in glutamine. , Nippon Shokuhin Kogyo Gakkaishi 39(1), 64-67.
- 52.Yamanaka H, Chachin K, Ogata K. (1971) Studies on the metabolism of free amino acids during maturation and ripening of tomato fruits II. Changes of the activities of glutamic acid decarboxylase and glutamic acid dehydrogenase in tomato fruits during maturation and ripening. , Journal of the Japanese Society for Horticultural Science 40, 287-291.
- 53.Yamanaka H, Chachin K, Ogata K. (1972) Studies on the Metabolism of Free Amino Acids during Maturation and Ripening of Tomato Fruits III. Metabolism of Glutamic Acid and γ-aminobutyric Acid during the Ripening of Tomato Fruits. , Journal of the Japanese Society for Horticultural Science 41, 317-321.
- 54.Saijo R, Nagata M, Ishi G No. (1989) . Proceedings of 5th International Control- led Atmosphere Research Conference , Wenatchee, WA, USA: 151, 151.
- 55.Lopez-Quiroga E, Prosapio V, P J Fryer, I T Norton, Bakalis S. (2019) Model discrimination for drying and rehydration kinetics of freeze-dried tomatoes. , Journal of Food Process Engineering 43, 13192-10.
Cited by (5)
This article has been cited by 5 scholarly works according to:
Citing Articles:
Applied Sciences (2025) OpenAlex
Tea Petković, E. Galić, K. Radić, Nikolina Golub, J. Jablan et al. - Applied Sciences (2025) Semantic Scholar
Asian Journal of Science Technology Engineering and Art (2024) OpenAlex
Bioactive Carbohydrates and Dietary Fibre (2023) Crossref
Bioactive Carbohydrates and Dietary Fibre (2023) OpenAlex
Saida Meziani, Amel Aissani, Ilham Khemis, B. Dave Oomah, Farid Zaidi - Bioactive Carbohydrates and Dietary Fibre (2023) Semantic Scholar